J Gastric Cancer.
2020 Mar;20(1):95-105. 10.5230/jgc.2020.20.e9.
The Influence of Bcl-3 Expression on Cell Migration and Chemosensitivity of Gastric Cancer Cells via Regulating Hypoxia-Induced Protective Autophagy
- Affiliations
-
- 1Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, National Clinical Research Center for Digestive Diseases, Beijing, China. zhangzht@ccmu.edu.cn
- 2Department of General Surgery, Beijing Jishuitan Hospital, The 4th Medical College of Peking University, Beijing, China.
- KMID: 2471929
- DOI: http://doi.org/10.5230/jgc.2020.20.e9
Abstract
- PURPOSE
Gastric cancer is a highly metastatic malignant tumor, often characterized by chemoresistance and high mortality. In the present study, we aimed to investigate the role of B-cell lymphoma 3 (Bcl-3) protein on cell migration and chemosensitivity of gastric cancer.
MATERIALS AND METHODS
The gastric cancer cell lines, AGS and NCI-N87, were used for the in vitro studies and the in vivo studies were performed using BALB/c nude mice. Western blotting, wound healing assay, Cell Counting Kit-8 assay, immunohistochemistry, and terminal deoxynucleotidyl transferase dUTP nick end labeling assay were used to evaluate the role of Bcl-3 in gastric cancer.
RESULTS
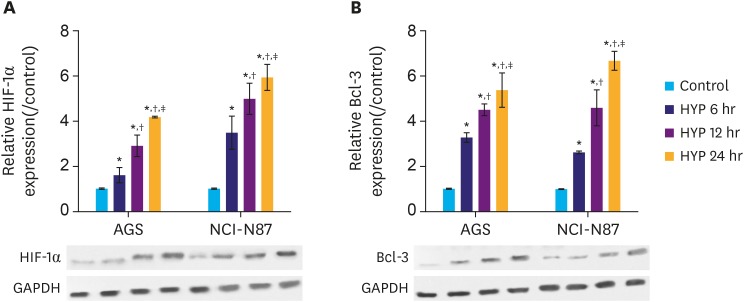
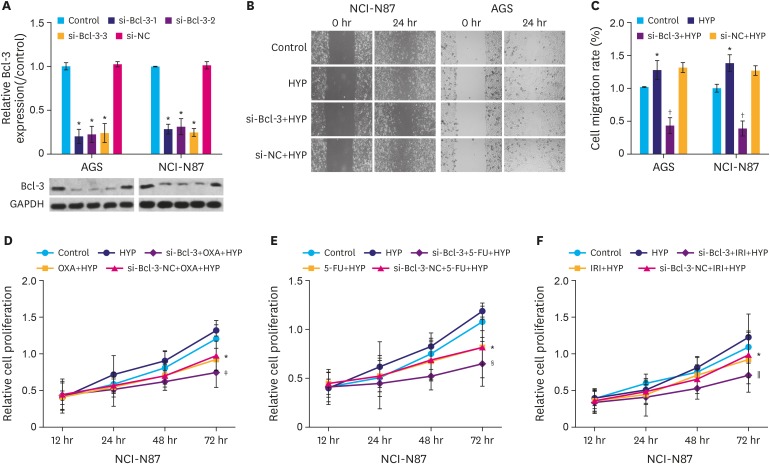
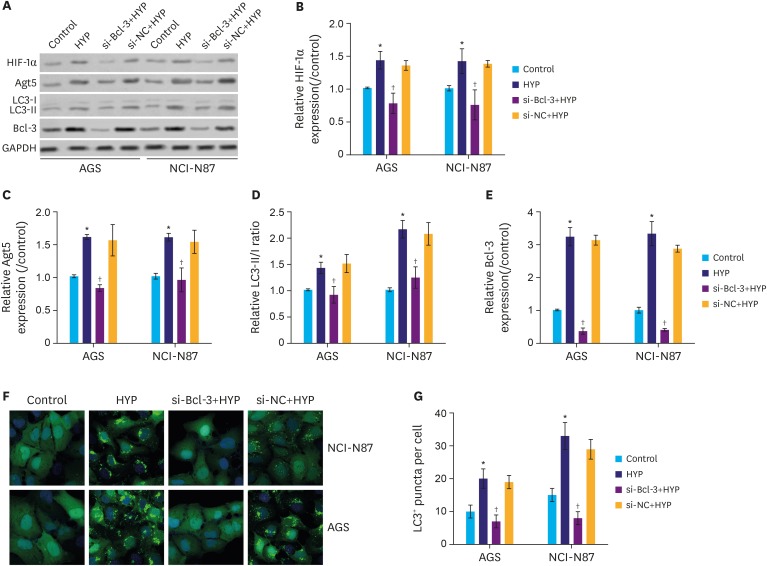
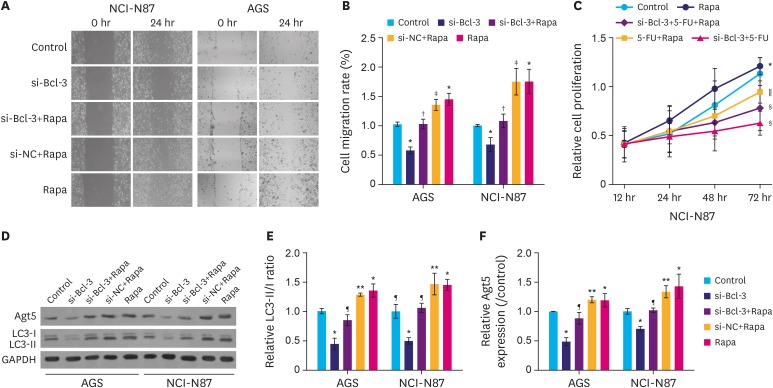
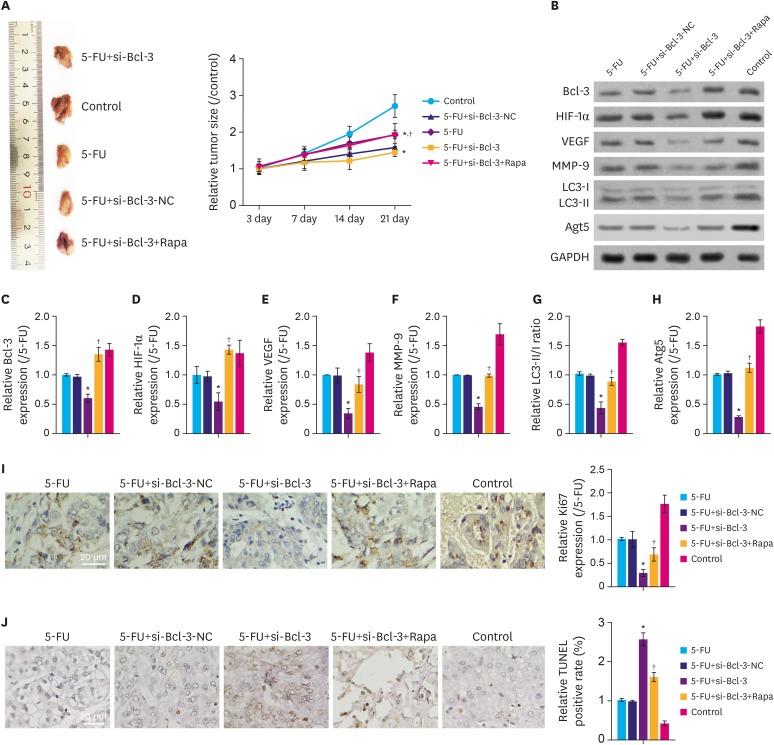
We found that the protein expression of hypoxia (HYP)-inducible factor-1α and Bcl-3 were markedly upregulated under hypoxic conditions in both AGS and NCI-N87 cells in a time-dependent manner. Interestingly, small interfering RNA-mediated knockdown of Bcl-3 expression affected the migration and chemosensitivity of the gastric cancer cells. AGS and NCI-N87 cells transfected with si-RNA-Bcl-3 (si-Bcl-3) showed significantly reduced migratory ability and increased chemosensitivity to oxaliplatin, 5-fluorouracil, and irinotecan. In addition, si-Bcl-3 restored the autophagy induced by HYP. Further, the protective role of si-Bcl-3 on the gastric cancer cells could be reversed by the autophagy inducer, rapamycin. Importantly, the in vivo xenograft tumor experiments showed similar results.
CONCLUSIONS
Our present study reveals that Bcl-3 knockdown inhibits cell migration and chemoresistance of gastric cancer cells through restoring HYP-induced autophagy.
Keyword
MeSH Terms
Figure
Reference
-
1. Choi H, Chang JW, Jung YK. Peroxiredoxin 6 interferes with TRAIL-induced death-inducing signaling complex formation by binding to death effector domain caspase. Cell Death Differ. 2011; 18:405–414. PMID: 20829884.
Article2. Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011; 364:656–665. PMID: 21323543.
Article3. Wulczyn FG, Naumann M, Scheidereit C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-kappa B. Nature. 1992; 358:597–599. PMID: 1501714.4. Hatada EN, Nieters A, Wulczyn FG, Naumann M, Meyer R, Nucifora G, et al. The ankyrin repeat domains of the NF-kappa B precursor p105 and the protooncogene bcl-3 act as specific inhibitors of NF-kappa B DNA binding. Proc Natl Acad Sci U S A. 1992; 89:2489–2493. PMID: 1532257.
Article5. Legge DN, Shephard AP, Collard TJ, Greenhough A, Chambers AC, Clarkson RW, et al. BCL-3 promotes a cancer stem cell phenotype by enhancing β-catenin signalling in colorectal tumour cells. Dis Model Mech. 2019; 12:dmm037697. PMID: 30792270.
Article6. Niu Y, Yang X, Chen Y, Zhang L, Jin X, Tang Y, et al. BCL3 expression is a potential prognostic and predictive biomarker in acute myeloid leukemia of FAB subtype M2. Pathol Oncol Res. 2019; 25:541–548. PMID: 30357752.
Article7. Wang H, Xiong M, Hu Y, Sun Y, Ma Q. MicroRNA-19b inhibits proliferation of gastric cancer cells by targeting B-cell CLL/lymphoma 3. Oncol Rep. 2016; 36:2079–2086. PMID: 27572553.
Article8. Rhoads MG, Kandarian SC, Pacelli F, Doglietto GB, Bossola M. Expression of NF-kappaB and IkappaB proteins in skeletal muscle of gastric cancer patients. Eur J Cancer. 2010; 46:191–197. PMID: 19857958.9. Bulajic M, Stimec B, Ille T, Jesenofsky R, Kecmanovic D, Pavlov M, et al. PCR detection of helicobacter pylori genome in colonic mucosa: normal and malignant. Prilozi. 2007; 28:25–38. PMID: 18356777.10. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003; 3:721–732. PMID: 13130303.
Article11. Legge DN, Shephard AP, Collard TJ, Greenhough A, Chambers AC, Clarkson RW, et al. BCL-3 promotes a cancer stem cell phenotype by enhancing β-catenin signalling in colorectal tumour cells. Dis Model Mech. 2019; 12:dmm037697. PMID: 30792270.
Article12. Che J, Wang W, Huang Y, Zhang L, Zhao J, Zhang P, et al. miR-20a inhibits hypoxia-induced autophagy by targeting ATG5/FIP200 in colorectal cancer. Mol Carcinog. 2019; 58:1234–1247. PMID: 30883936.
Article13. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–132. PMID: 26808342.
Article14. Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014; 9:47–71. PMID: 23937437.
Article15. Che J, Wang W, Huang Y, Zhang L, Zhao J, Zhang P, et al. miR-20a inhibits hypoxia-induced autophagy by targeting ATG5/FIP200 in colorectal cancer. Mol Carcinog. 2019; 58:1234–1247. PMID: 30883936.
Article16. Pan Y, Shao D, Zhao Y, Zhang F, Zheng X, Tan Y, et al. Berberine reverses hypoxia-induced chemoresistance in breast cancer through the inhibition of AMPK- HIF-1α. Int J Biol Sci. 2017; 13:794–803. PMID: 28656004.
Article17. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985). 2000; 88:1474–1480. PMID: 10749844.
Article18. Cao Q, Liu F, Ji K, Liu N, He Y, Zhang W, et al. MicroRNA-381 inhibits the metastasis of gastric cancer by targeting TMEM16A expression. J Exp Clin Cancer Res. 2017; 36:29. PMID: 28193228.
Article19. Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS Jr. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000; 19:1123–1131. PMID: 10713699.20. Ahlqvist K, Saamarthy K, Syed Khaja AS, Bjartell A, Massoumi R. Expression of Id proteins is regulated by the Bcl-3 proto-oncogene in prostate cancer. Oncogene. 2013; 32:1601–1608. PMID: 22580608.
Article21. Maldonado V, Espinosa M, Pruefer F, Patiño N, Ceballos-Canciono G, Urzua U, et al. Gene regulation by BCL3 in a cervical cancer cell line. Folia Biol (Praha). 2010; 56:183–193. PMID: 20974051.22. Huo J, Chen X, Zhang H, Hu Y, Jiang Y, Liu S, et al. Bcl-3 promotes proliferation and chemosensitivity in BL1 subtype of TNBC cells. Acta Biochim Biophys Sin (Shanghai). 2018; 50:1141–1149. PMID: 30289427.
Article23. Zou Y, Uddin MM, Padmanabhan S, Zhu Y, Bu P, Vancura A, et al. The proto-oncogene Bcl3 induces immune checkpoint PD-L1 expression, mediating proliferation of ovarian cancer cells. J Biol Chem. 2018; 293:15483–15496. PMID: 30135206.
Article24. Long TY, Jing R, Kuang F, Huang L, Qian ZX, Yang TL. CIRBP protects H9C2 cells against myocardial ischemia through inhibition of NF-κB pathway. Braz J Med Biol Res. 2017; 50:e5861–e5861. PMID: 28355355.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dexmedetomidine alleviates CoCl2-induced hypoxic cellular damage in INS-1 cells by regulating autophagy
- Propofol Attenuates Hypoxia/Reoxygenation-Induced Apoptosis and Autophagy in HK-2 Cells by Inhibiting JNK Activation
- Relationship between the Expression of Apoptosis-Related Proteins and Chemosensitivity in Gastric Cancer Cell Lines
- Hepatitis B virus X Protein Promotes Liver Cancer Progression through Autophagy Induction in Response to TLR4 Stimulation
- Hepatitis B virus X Protein Promotes Liver Cancer Progression through Autophagy Induction in Response to TLR4 Stimulation