Korean J Physiol Pharmacol.
2020 Mar;24(2):173-183. 10.4196/kjpp.2020.24.2.173.
The optimal model of reperfusion injury in vitro using H9c2 transformed cardiac myoblasts
- Affiliations
-
- 1Department of Pharmacology, University of Ulsan College of Medicine, Seoul 05505, Korea. kimyh@amc.seoul.kr
- 2Bio-Medical Institute of Technology, University of Ulsan, Seoul 05505, Korea.
- 3Stem Cell Immunomodulation Research Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 4Department of Medical Science, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 5Asan Institute for Life Sciences, Asan Medical Center, Seoul 05505, Korea. cwwoo@amc.seoul.kr
- KMID: 2471039
- DOI: http://doi.org/10.4196/kjpp.2020.24.2.173
Abstract
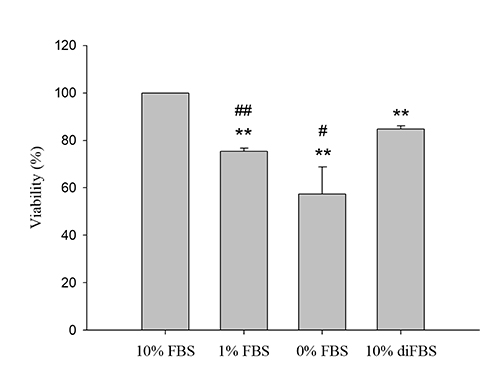
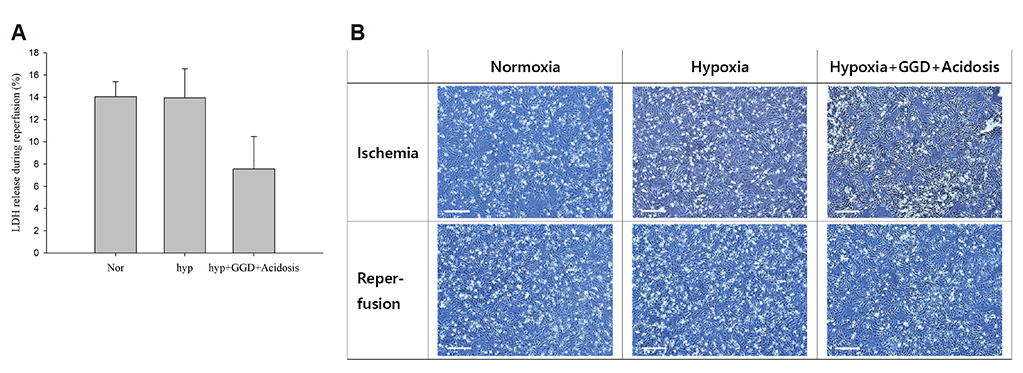
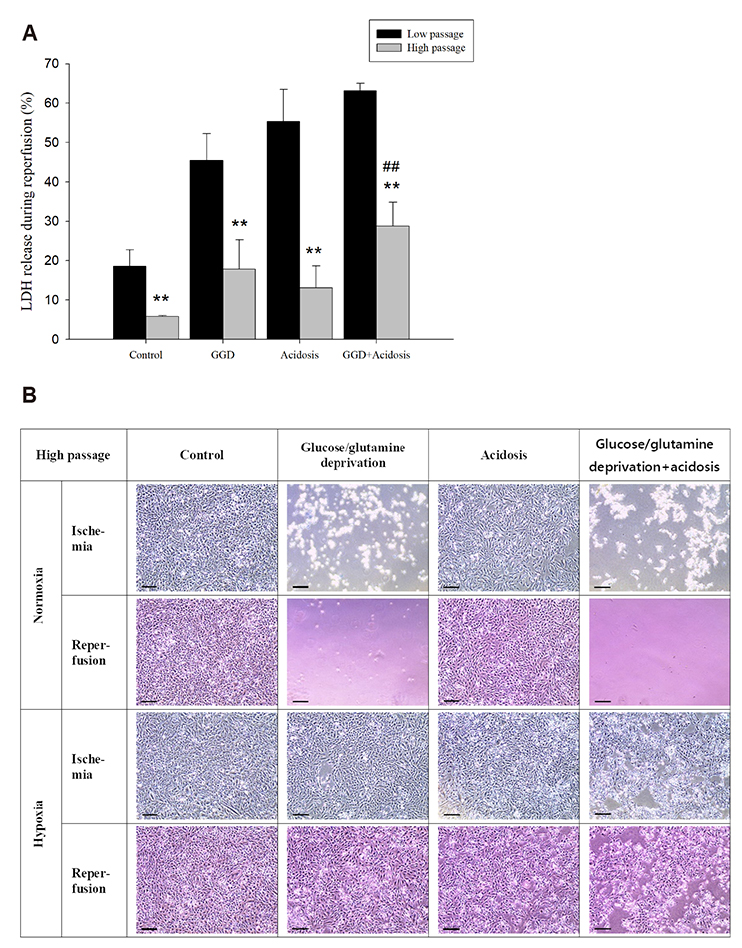
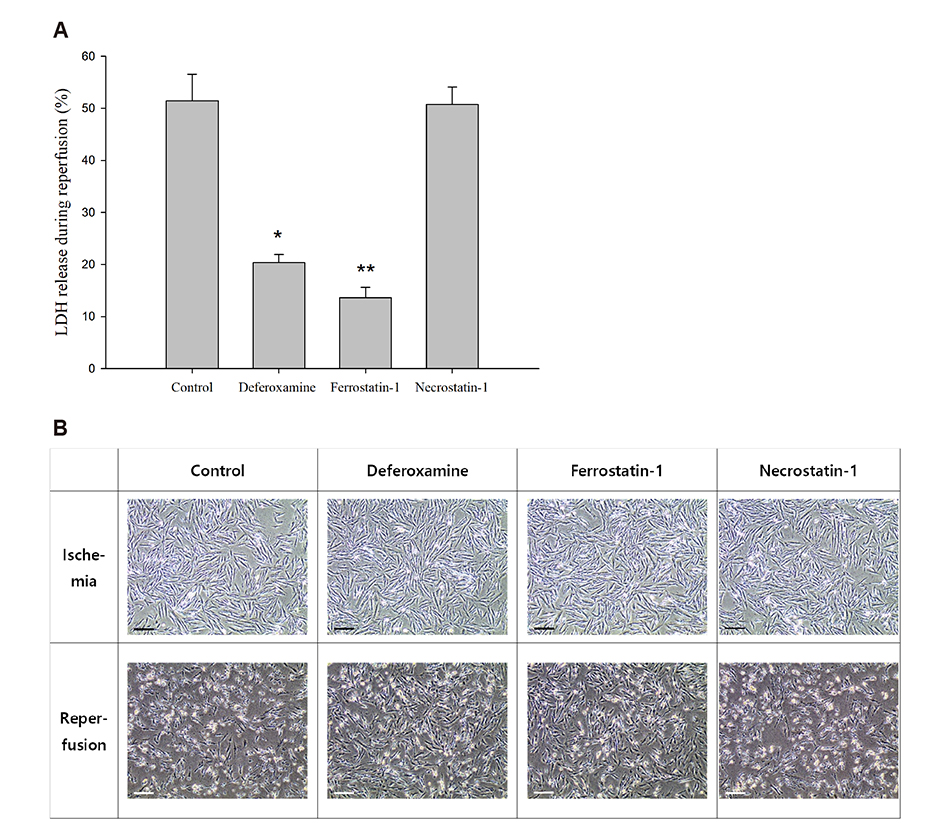
- An in vitro model for ischemia/reperfusion injury has not been well-established. We hypothesized that this failure may be caused by serum deprivation, the use of glutamine-containing media, and absence of acidosis. Cell viability of H9c2 cells was significantly decreased by serum deprivation. In this condition, reperfusion damage was not observed even after simulating severe ischemia. However, when cells were cultured under 10% dialyzed FBS, cell viability was less affected compared to cells cultured under serum deprivation and reperfusion damage was observed after hypoxia for 24 h. Reperfusion damage after glucose or glutamine deprivation under hypoxia was not significantly different from that after hypoxia only. However, with both glucose and glutamine deprivation, reperfusion damage was significantly increased. After hypoxia with lactic acidosis, reperfusion damage was comparable with that after hypoxia with glucose and glutamine deprivation. Although high-passage H9c2 cells were more resistant to reperfusion damage than low-passage cells, reperfusion damage was observed especially after hypoxia and acidosis with glucose and glutamine deprivation. Cell death induced by reperfusion after hypoxia with acidosis was not prevented by apoptosis, autophagy, or necroptosis inhibitors, but significantly decreased by ferrostatin-1, a ferroptosis inhibitor, and deferoxamine, an iron chelator. These data suggested that in our SIR model, cell death due to reperfusion injury is likely to occur via ferroptosis, which is related with ischemia/reperfusion-induced cell death in vivo. In conclusion, we established an optimal reperfusion injury model, in which ferroptotic cell death occurred by hypoxia and acidosis with or without glucose/glutamine deprivation under 10% dialyzed FBS.
MeSH Terms
Figure
Reference
-
1. King LM, Opie LH. Glucose and glycogen utilisation in myocardial ischemia--changes in metabolism and consequences for the myocyte. Mol Cell Biochem. 1998; 180:3–26.2. Hearse DJ. Reperfusion of the ischemic myocardium. J Mol Cell Cardiol. 1977; 9:605–616.
Article3. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013; 123:92–100.
Article4. Lee JW, Lee HK, Kim HW, Kim YH. Effects of pH, buffer system and lactate on the simulated ischemia-reperfusion injury of H9c2 cardiac myocytes. Korean J Physiol Pharmacol. 2007; 11:45–55.5. Yang GZ, Xue FS, Liu YY, Li HX, Liu Q, Liao X. Feasibility analysis of oxygen-glucose deprivation-nutrition resumption on H9c2 cells in vitro models of myocardial ischemia-reperfusion injury. Chin Med J (Engl). 2018; 131:2277–2286.6. Lee SB, Kim JJ, Kim TW, Kim BS, Lee MS, Yoo YD. Serum deprivation-induced reactive oxygen species production is mediated by Romo1. Apoptosis. 2010; 15:204–218.
Article7. Khogali SE, Harper AA, Lyall JA, Rennie MJ. Effects of L-glutamine on post-ischaemic cardiac function: protection and rescue. J Mol Cell Cardiol. 1998; 30:819–827.8. Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015; 59:298–308.
Article9. Steg PG, James SK, Gersh BJ. evidence-based recommendations, ensuring optimal patient management. Heart. 2013; 99:1156–1157.10. Bates ER, Jacobs AK. Time to treatment in patients with STEMI. N Engl J Med. 2013; 369:889–892.
Article11. Bond JM, Herman B, Lemasters JJ. Protection by acidotic pH against anoxia/reoxygenation injury to rat neonatal cardiac myocytes. Biochem Biophys Res Commun. 1991; 179:798–803.
Article12. Li L, Hao Y, Zhao Y, Wang H, Zhao X, Jiang Y, Gao F. Ferroptosis is associated with oxygen-glucose deprivation/reoxygenation-induced Sertoli cell death. Int J Mol Med. 2018; 41:3051–3062.
Article13. Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, Zhang F, Ning S, Yao J, Tian X. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019; 26:2284–2299.
Article14. Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, Cheng Q, Zhang P, Dai W, Chen J, Yang F, Yang HT, Linkermann A, Gu W, Min J, Wang F. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019; 116:2672–2680.
Article15. Bonavita F, Stefanelli C, Giordano E, Columbaro M, Facchini A, Bonafè F, Caldarera CM, Guarnieri C. H9c2 cardiac myoblasts undergo apoptosis in a model of ischemia consisting of serum deprivation and hypoxia: inhibition by PMA. FEBS Lett. 2003; 536:85–91.
Article16. Kues WA, Anger M, Carnwath JW, Paul D, Motlik J, Niemann H. Cell cycle synchronization of porcine fetal fibroblasts: effects of serum deprivation and reversible cell cycle inhibitors. Biol Reprod. 2000; 62:412–419.17. Howard MK, Burke LC, Mailhos C, Pizzey A, Gilbert CS, Lawson WD, Collins MK, Thomas NS, Latchman DS. Cell cycle arrest of proliferating neuronal cells by serum deprivation can result in either apoptosis or differentiation. J Neurochem. 1993; 60:1783–1791.
Article18. Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao R. Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation. 2004; 109:898–903.
Article19. Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, Zimmerman LJ, Liebler DC, Slebos RJ, Lorkiewicz PK, Higashi RM, Fan TW, Dang CV. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012; 15:110–121.20. Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, Rabinowitz JD. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol. 2013; 9:712.
Article21. DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007; 104:19345–19350.
Article22. Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, Kimmelman AC. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013; 496:101–105.
Article23. Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, Jiang X. Role of Mitochondria in Ferroptosis. Mol Cell. 2019; 73:354–363.e3.
Article24. Myers RA. Hyperbaric oxygen therapy for trauma: crush injury, compartment syndrome, and other acute traumatic peripheral ischemias. Int Anesthesiol Clin. 2000; 38:139–151.
Article25. Kitakaze M, Weisfeldt ML, Marban E. Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts. J Clin Invest. 1988; 82:920–927.
Article26. Inserte J, Barba I, Hernando V, Abellán A, Ruiz-Meana M, Rodríguez-Sinovas A, Garcia-Dorado D. Effect of acidic reperfusion on prolongation of intracellular acidosis and myocardial salvage. Cardiovasc Res. 2008; 77:782–790.
Article27. Khacho M, Tarabay M, Patten D, Khacho P, MacLaurin JG, Guadagno J, Bergeron R, Cregan SP, Harper ME, Park DS, Slack RS. Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat Commun. 2014; 5:3550.
Article28. Williams RE, Zweier JL, Flaherty JT. Treatment with deferoxamine during ischemia improves functional and metabolic recovery and reduces reperfusion-induced oxygen radical generation in rabbit hearts. Circulation. 1991; 83:1006–1014.
Article29. Elihu N, Anandasbapathy S, Frishman WH. Chelation therapy in cardiovascular disease: ethylenediaminetetraacetic acid, deferoxamine, and dexrazoxane. J Clin Pharmacol. 1998; 38:101–105.
Article30. Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016; 23:369–379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of pH, Buffer System and Lactate on the Simulated Ischemia-reperfusion Injury of H9c2 Cardiac Myocytes
- The Different Expression Patterns of HSP22, a Late Embryogenesis Abundant-like Protein, in Hypertrophic H9C2 Cells Induced by NaCl and Angiotensin II
- Ischemic postconditioning protects cardiomyocytes against ischemia/reperfusion injury by inducing MIP2
- Studies on Differentiation of Cardiac Myoblast Induced by Co-culture with Isolated Neonatal Rat Cardiac Myocytes
- Benzoylaconine improves mitochondrial function in oxygenglucose deprivation and reperfusion-induced cardiomyocyte injury by activation of the AMPK/PGC-1 axis