Korean J Physiol Pharmacol.
2022 Sep;26(5):325-333. 10.4196/kjpp.2022.26.5.325.
Benzoylaconine improves mitochondrial function in oxygenglucose deprivation and reperfusion-induced cardiomyocyte injury by activation of the AMPK/PGC-1 axis
- Affiliations
-
- 1Department of Cardiology, Hebi People’s Hospital, Hebi 458030, China
- 2Department of Cardiovascular Medicine, Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou 310022, China
- 3Department of Geriatrics, Hubin Street Community Health Service Center, Hangzhou 310000, China
- KMID: 2532762
- DOI: http://doi.org/10.4196/kjpp.2022.26.5.325
Abstract
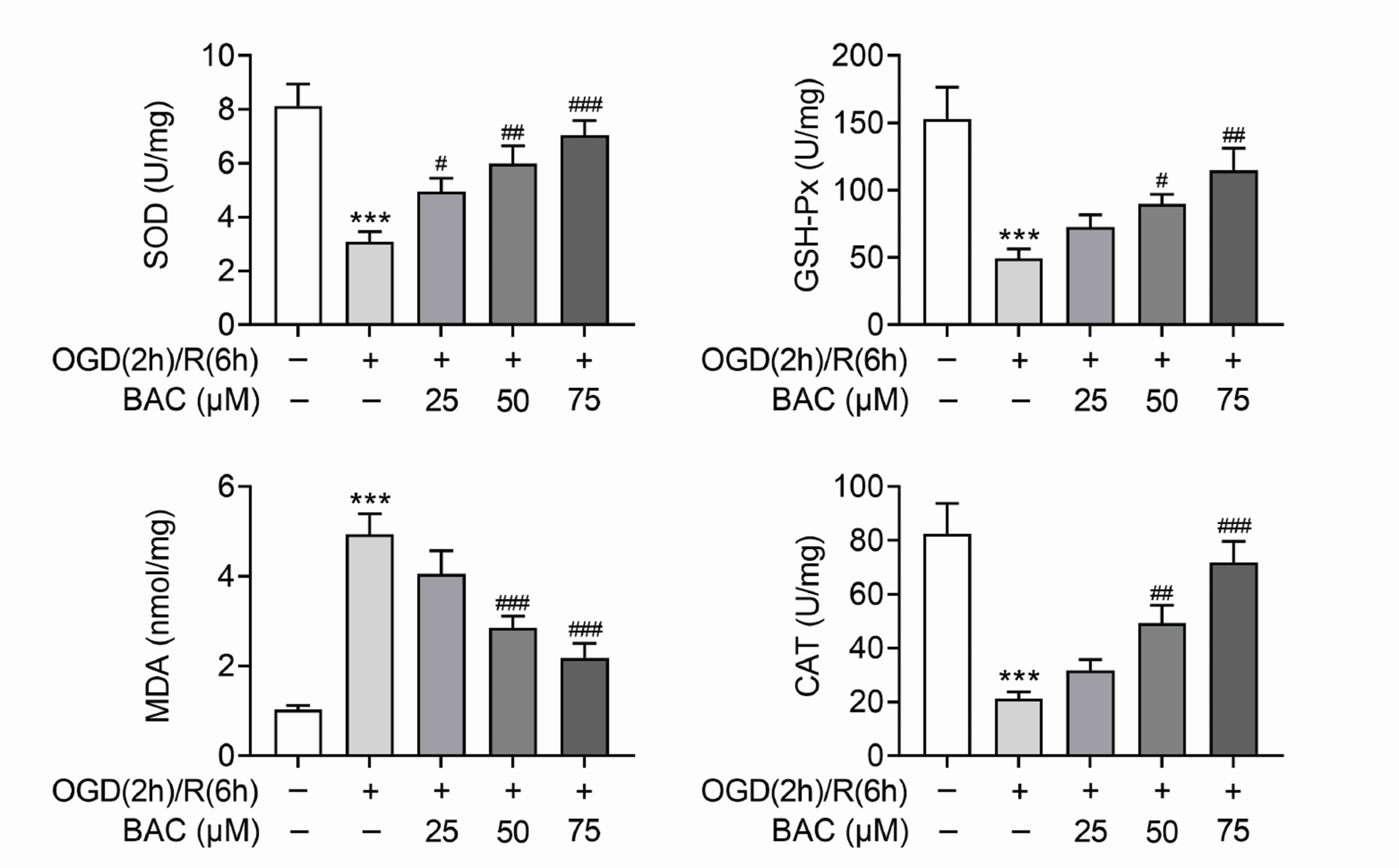
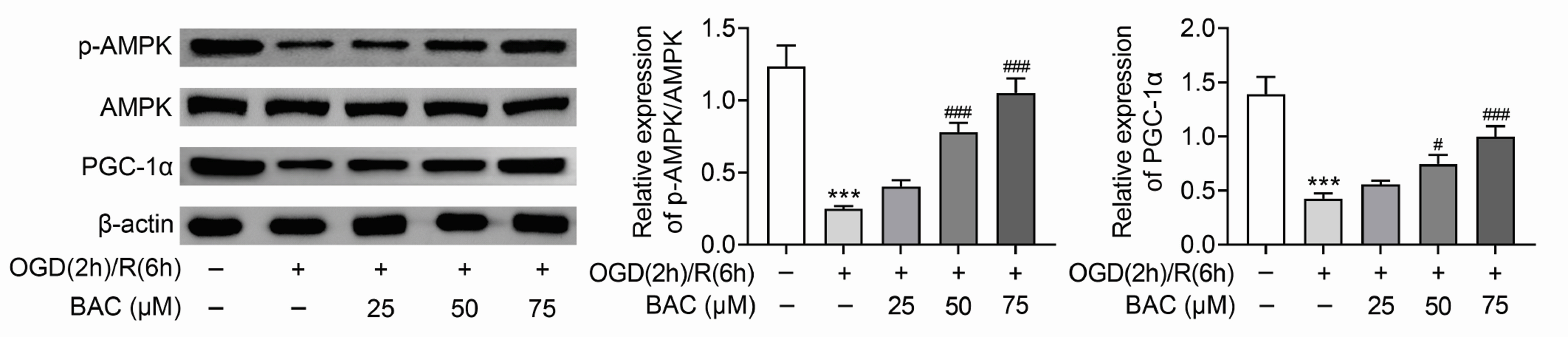
- Heart failure (HF) has become one of the severe public health problems. The detailed role of mitochondrial function in HF was still unclear. Benzoylaconine (BAC) is a traditional Chinese medicine, but its role in HF still needs to be explored. In this study, oxygen-glucose deprivation and reperfusion (OGD/R) was executed to mimic the injury of H9C2 cells in HF. The viability of H9C2 cells was assessed via MTT assay. OGD/R treatment markedly decreased the viability of H9C2 cells, but BAC treatment evidently increased the viability of OGD/R-treated H9C2 cells. The apoptosis of H9C2 was enhanced by OGD/R treatment but suppressed by BAC treatment. The mitochondrial membrane potential was evaluated via JC-1 assay. BAC improved the mitochondrial function and suppressed oxidative stress in OGD/R-treated H9C2 cells. Moreover, Western blot analysis revealed that the protein expression of p-AMPK and PGC-1α were reduced in OGD/R-treated H9C2 cells, which was reversed by BAC. Rescue assays indicated that AMPK attenuation reversed the BAC-mediated protective effect on OGD/R-treated cardiomyocytes. Moreover, BAC alleviated myocardial injury in vivo. In a word, BAC modulated the mitochondrial function in OGD/R-induced cardiomyocyte injury by activation of the AMPK/PGC-1 axis. The findings might provide support for the application of BAC in the treatment of HF.
Figure
Reference
-
1. Mensah GA, Roth GA, Fuster V. 2019; The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. 74:2529–2532. DOI: 10.1016/j.jacc.2019.10.009. PMID: 31727292.2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, et al. 2016; 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 18:891–975. DOI: 10.1093/eurheartj/ehw128. PMID: 27206819.
Article3. Chang IH, Lin LJ, Wu RC. 2022; Acute heart failure from a rapidly progressing large tumor in the right atrium. Signa Vitae. 18:136–138. https://doi.org/10.22514/sv.2021.095. DOI: 10.22514/sv.2021.095.
Article4. Truby LK, Rogers JG. 2020; Advanced heart failure: epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail. 8:523–536. DOI: 10.1016/j.jchf.2020.01.014. PMID: 32535126.5. Jiang H, Xing J, Fang J, Wang L, Wang Y, Zeng L, Li Z, Liu R. 2020; Tilianin protects against ischemia/reperfusion-induced myocardial injury through the inhibition of the Ca2+/calmodulin-dependent protein kinase II-dependent apoptotic and inflammatory signaling pathways. Biomed Res Int. 2020:5939715. DOI: 10.1155/2020/5939715. PMID: 33102583. PMCID: PMC7568786.
Article6. Geng Y, Hu Y, Wang H, Shi S, Shi J, Qiu Z. 2017; Deficiency of interfibrillar mitochondria in post-acute myocardial infarction heart failure. Pak J Pharm Sci. 30(3(Special)):1089–1094.7. Niu X, Pu S, Ling C, Xu J, Wang J, Sun S, Yao Y, Zhang Z. 2020; lncRNA Oip5-as1 attenuates myocardial ischaemia/reperfusion injury by sponging miR-29a to activate the SIRT1/AMPK/PGC1α pathway. Cell Prolif. 53:e12818. DOI: 10.1111/cpr.12818. PMID: 32468629. PMCID: PMC7309946.
Article8. Zhou B, Tian R. 2018; Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 128:3716–3726. DOI: 10.1172/JCI120849. PMID: 30124471. PMCID: PMC6118589.
Article9. Tan G, Liao W, Dong X, Yang G, Zhu Z, Li W, Chai Y, Lou Z. 2012; Metabonomic profiles delineate the effect of traditional Chinese medicine sini decoction on myocardial infarction in rats. PLoS One. 7:e34157. DOI: 10.1371/journal.pone.0034157. PMID: 22493681. PMCID: PMC3320902. PMID: 4c6ec630c9434d768502a4722b9bfb21.
Article10. Liu J, Li Q, Yin Y, Liu R, Xu H, Bi K. 2014; Ultra-fast LC-ESI-MS/MS method for the simultaneous determination of six highly toxic Aconitum alkaloids from Aconiti kusnezoffii radix in rat plasma and its application to a pharmacokinetic study. J Sep Sci. 37:171–178. DOI: 10.1002/jssc.201300775. PMID: 24170571.
Article11. Deng XH, Liu JJ, Sun XJ, Dong JC, Huang JH. 2019; Benzoylaconine induces mitochondrial biogenesis in mice via activating AMPK signaling cascade. Acta Pharmacol Sin. 40:658–665. DOI: 10.1038/s41401-018-0174-8. PMID: 30315253. PMCID: PMC6786398.
Article12. Zhou C, Gao J, Ji H, Li W, Xing X, Liu D, Guo Q, Zhou L, Jing F. 2021; Benzoylaconine modulates LPS-induced responses through inhibition of toll-like receptor-mediated NF-κB and MAPK signaling in RAW264.7 cells. Inflammation. 44:2018–2032. DOI: 10.1007/s10753-021-01478-z. PMID: 34272638.
Article13. Zhang H, Sun S, Zhang W, Xie X, Zhu Z, Chai Y, Zhang G. 2016; Biological activities and pharmacokinetics of aconitine, benzoylaconine, and aconine after oral administration in rats. Drug Test Anal. 8:839–846. DOI: 10.1002/dta.1858. PMID: 26360128.
Article14. Zhang X, Gao Y, Wu H, Mao Y, Qi Y. 2022; LncRNA HOX transcript antisense RNA mitigates cardiac function injury in chronic heart failure via regulating microRNA-30a-5p to target KDM3A. J Cell Mol Med. 26:1473–1485. DOI: 10.1111/jcmm.17160. PMID: 35083842. PMCID: PMC8899154.
Article15. van der Bliek AM, Sedensky MM, Morgan PG. 2017; Cell biology of the mitochondrion. Genetics. 207:843–871. Erratum in: Genetics. 2018;208:1673. DOI: 10.1534/genetics.117.300262. PMID: 29097398. PMCID: PMC5676242.
Article16. Kumar AA, Kelly DP, Chirinos JA. 2019; Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 139:1435–1450. DOI: 10.1161/CIRCULATIONAHA.118.036259. PMID: 30856000. PMCID: PMC6414077.
Article17. Yu H, Zhang F, Yan P, Zhang S, Lou Y, Geng Z, Li Z, Zhang Y, Xu Y, Lu Y, Chen C, Wang D, Zhu W, Hu X, Wang J, Zhuang T, Zhang Y, Wu G, Liu J, Zeng C, et al. 2021; LARP7 protects against heart failure by enhancing mitochondrial biogenesis. Circulation. 143:2007–2022. DOI: 10.1161/CIRCULATIONAHA.120.050812. PMID: 33663221.
Article18. Kiyuna LA, Albuquerque RPE, Chen CH, Mochly-Rosen D, Ferreira JCB. 2018; Targeting mitochondrial dysfunction and oxidative stress in heart failure: challenges and opportunities. Free Radic Biol Med. 129:155–168. DOI: 10.1016/j.freeradbiomed.2018.09.019. PMID: 30227272. PMCID: PMC6309415.
Article19. Chen X, Wang Q, Shao M, Ma L, Guo D, Wu Y, Gao P, Wang X, Li W, Li C, Wang Y. 2019; Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway. Biomed Pharmacother. 120:109487. DOI: 10.1016/j.biopha.2019.109487. PMID: 31577975.20. Zhang Q, Guo D, Wang Y, Wang X, Wang Q, Wu Y, Li C, Wang W, Wang Y. 2020; Danqi pill protects against heart failure post-acute myocardial infarction via HIF-1α/PGC-1α mediated glucose metabolism pathway. Front Pharmacol. 11:458. DOI: 10.3389/fphar.2020.00458. PMID: 32372956. PMCID: PMC7187888. PMID: 6091ca491bf64d798cbc439c4fd89cc0.
Article21. Wen J, Zhang L, Liu H, Wang J, Li J, Yang Y, Wang Y, Cai H, Li R, Zhao Y. 2019; Salsolinol attenuates doxorubicin-induced chronic heart failure in rats and improves mitochondrial function in H9c2 cardiomyocytes. Front Pharmacol. 10:1135. DOI: 10.3389/fphar.2019.01135. PMID: 31680945. PMCID: PMC6797600.
Article22. Li Y, Chen Y. 2019; AMPK and autophagy. Adv Exp Med Biol. 1206:85–108. DOI: 10.1007/978-981-15-0602-4_4. PMID: 31776981.
Article23. Di W, Lv J, Jiang S, Lu C, Yang Z, Ma Z, Hu W, Yang Y, Xu B. 2018; PGC-1: the energetic regulator in cardiac metabolism. Curr Issues Mol Biol. 28:29–46. DOI: 10.21775/cimb.028.029. PMID: 29388552.
Article24. Cardoso SM, Correia SC, Carvalho C, Moreira PI. 2017; Mitochondria in Alzheimer's disease and diabetes-associated neurodegeneration: license to heal! Handb Exp Pharmacol. 240:281–308. DOI: 10.1007/164_2017_3. PMID: 28251365.
Article25. Chen J, Wong HS, Leong PK, Leung HY, Chan WM, Ko KM. 2017; Ursolic acid induces mitochondrial biogenesis through the activation of AMPK and PGC-1 in C2C12 myotubes: a possible mechanism underlying its beneficial effect on exercise endurance. Food Funct. 8:2425–2436. DOI: 10.1039/C7FO00127D. PMID: 28675237.
Article26. Zhang Q, Liang XC. 2019; Effects of mitochondrial dysfunction via AMPK/PGC-1 α signal pathway on pathogenic mechanism of diabetic peripheral neuropathy and the protective effects of Chinese medicine. Chin J Integr Med. 25:386–394. DOI: 10.1007/s11655-018-2579-0. PMID: 30656599.
Article27. Sun JP, Shi L, Wang F, Qin J, Ke B. 2022; Modified Linggui Zhugan Decoction (加味苓桂术甘汤) ameliorates glycolipid metabolism and inflammation via PI3K-Akt/mTOR-S6K1/AMPK-PGC-1 α signaling pathways in obese type 2 diabetic rats. Chin J Integr Med. 28:52–59. English, Chinese. DOI: 10.1007/s11655-020-3285-2. PMID: 33211278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mitochondrial Dynamics in the Heart as a Novel Therapeutic Target for Cardioprotection
- Hypothermia protects against renal fibrosis after ischemia reperfusion injury
- Mechanism of Ischemia and Reperfusion Injury to the Heart: From the Viewpoint of Nitric Oxide and Mitochondria
- Mechanical Stretch-Induced Protection against Myocardial Ischemia-Reperfusion Injury Involves AMP-Activated Protein Kinase
- PGC1-alpha plays a role in hypothermic renal protection of renal fibrosis after acute kidney injury