Clin Exp Vaccine Res.
2020 Jan;9(1):40-47. 10.7774/cevr.2020.9.1.40.
Immunogenicity of a new, inactivated canine adenovirus type 2 vaccine for dogs
- Affiliations
-
- 1Viral Disease Research Division, Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, Gimcheon, Korea. yangdk@korea.kr
- KMID: 2470836
- DOI: http://doi.org/10.7774/cevr.2020.9.1.40
Abstract
- PURPOSE
We constructed a new canine adenovirus type 2 (CAV-2) vaccine candidate using the recently isolated Korean CAV-2 strain; we termed the vaccine APQA1701-40P and evaluated its safety and immunogenicity in dogs.
MATERIALS AND METHODS
To generate the anti-CAV-2 vaccine, APQA1701 was passaged 40 times in MDCK cells growing in medium containing 5 mM urea and the virus was inactivated using 0.05% (volume per volume) formaldehyde. Two vaccines were prepared by blending inactivated APQA1701-40P with two different adjuvants; both were intramuscularly injected (twice) into guinea pigs. The safety and immunogenicity of the Cabopol-adjuvanted vaccine were evaluated in seronegative dogs. The humoral responses elicited were measured using an indirect enzyme-linked immunosorbent assay (I-ELISA), and via a virus neutralization assay (VNA).
RESULTS
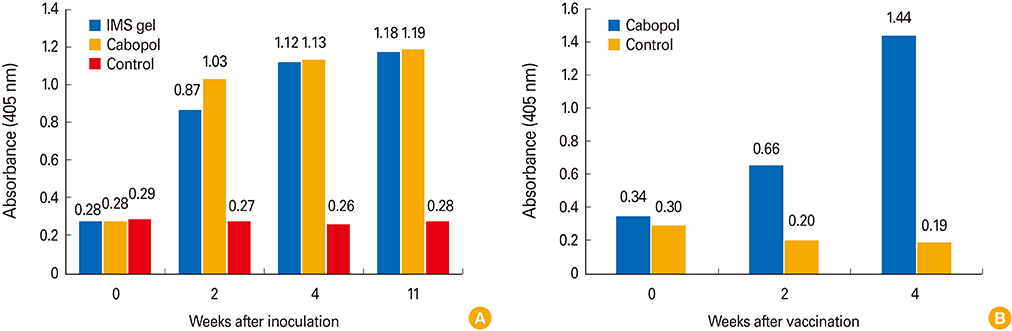
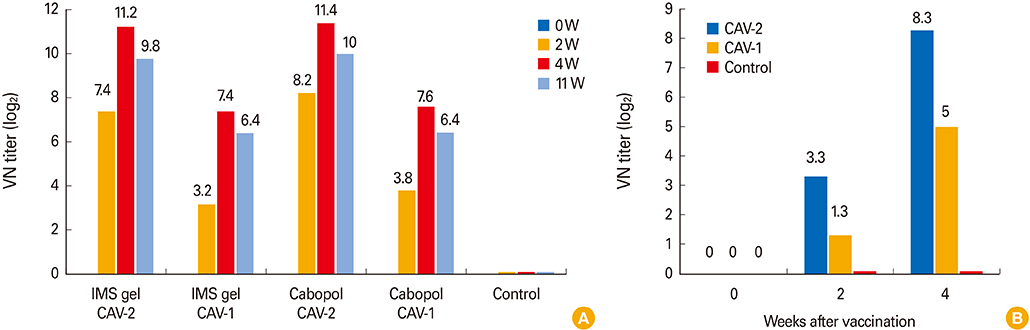
The new, inactivated CAV-2 vaccine strain, APQA1701-40P, lacked six amino acids of the E1b-19K protein. In guinea pigs, the Cabopol-adjuvanted vaccine afforded a slightly higher VNA titer and I-ELISA absorbance than an IMS gel-adjuvanted vaccine 4 weeks post-vaccination (p>0.05). Dogs inoculated with the former vaccine developed a significantly higher immune titer than non-vaccinated dogs.
CONCLUSION
The Cabopol-adjuvanted, inactivated CAV-2 vaccine was safe and induced a high VNA titer in dogs.
Keyword
MeSH Terms
Figure
Reference
-
1. Timurkan MO, Aydin H, Alkan F. Detection and molecular characterization of canine adenovirus type 2 (CAV-2) in dogs with respiratory tract symptoms in shelters in Turkey. Vet Arch. 2018; 88:467–479.
Article2. Hu RL, Huang G, Qiu W, Zhong ZH, Xia XZ, Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet Res Commun. 2001; 25:77–84.3. Russell WC. Adenoviruses: update on structure and function. J Gen Virol. 2009; 90:1–20.
Article4. Pehler-Harrington K, Khanna M, Waters CR, Henrickson KJ. Rapid detection and identification of human adenovirus species by adenoplex, a multiplex PCR-enzyme hybridization assay. J Clin Microbiol. 2004; 42:4072–4076.
Article5. Appel M, Carmichael LE, Robson DS. Canine adenovirus type 2-induced immunity to two canine adenoviruses in pups with maternal antibody. Am J Vet Res. 1975; 36:1199–1202.6. Goldstein T, Colegrove KM, Hanson M, Gulland FM. Isolation of a novel adenovirus from California sea lions Zalophus californianus. Dis Aquat Organ. 2011; 94:243–248.
Article7. Choi JW, Lee HK, Kim SH, et al. Canine adenovirus type 1 in a fennec fox (Vulpes zerda). J Zoo Wildl Med. 2014; 45:947–950.
Article8. Park NY, Lee MC, Kurkure NV, Cho HS. Canine adenovirus type 1 infection of a Eurasian river otter (Lutra lutra). Vet Pathol. 2007; 44:536–539.
Article9. Yoon SS, Byun JW, Park YI, Kim MJ, Bae YC, Song JY. Comparison of the diagnostic methods on the canine adenovirus type 2 infection. Basic Appl Pathol. 2010; 3:52–56.
Article10. Yang DK, Kim HH, Yoon SS, Lee H, Cho IS. Isolation and identification of canine adenovirus type 2 from a naturally infected dog in Korea. Korean J Vet Res. 2018; 58:177–182.
Article11. Yang DK, Kim HH, Yoon SS, Ji M, Cho IS. Incidence and sero-survey of canine adenovirus type 2 in various animal species. J Bacteriol Virol. 2018; 48:102–108.
Article12. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010; 33:492–503.
Article13. Mair KH, Koinig H, Gerner W, et al. Carbopol improves the early cellular immune responses induced by the modified-life vaccine Ingelvac PRRS(R) MLV. Vet Microbiol. 2015; 176:352–357.
Article14. Xu Y, Wang Q, Wei B, et al. Enhanced immune responses against japanese encephalitis virus infection using Japanese encephalitis live-attenuated virus adjuvanted with montanide GEL 01 ST in mice. Vector Borne Zoonotic Dis. 2019; 19:835–843.
Article15. Yang DK, Kim HH, Lee EJ, et al. Recharacterization of the canine adenovirus type 1 vaccine strain based on the biological and molecular properties. J Bacteriol Virol. 2019; 49:124–132.
Article16. Sira S, Abouhaidar MG, Liu YC, Campbell JB. Multiple reiteration of a 40-bp nucleotide sequence in the inverted terminal repeat of the genome of a canine adenovirus. Virology. 1987; 159:76–83.
Article17. Cavanagh HM, Gallagher CF, Spibey N. A mutant of canine adenovirus type 2 with a duplication of the E1a region exhibits altered expression of early region 4. J Gen Virol. 1991; 72:2121–2127.
Article18. White E, Cipriani R, Sabbatini P, Denton A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991; 65:2968–2978.
Article19. Subramanian T, Tarodi B, Govindarajan R, Boyd JM, Yoshida K, Chinnadurai G. Mutational analysis of the transforming and apoptosis suppression activities of the adenovirus E1B 175R protein. Gene. 1993; 124:173–181.
Article20. Tarodi B, Subramanian T, Chinnadurai G. Functional similarity between adenovirus e1b 19k gene and bcl2 oncogene: mutant complementation and suppression of cell-death induced by DNA-damaging agents. Int J Oncol. 1993; 3:467–472.21. Zhang J, Wang M, Zhou N, Shen Y, Li Y. Evaluation of carbopol as an adjuvant on the effectiveness of progressive atrophic rhinitis vaccine. Vaccine. 2018; 36:4477–4484.
Article22. Cornwell HJ, Koptopoulos G, Thompson H, McCandlish IA, Wright NG. Immunity to canine adenovirus respiratory disease: a comparison of attenuated CAV-1 and CAV-2 vaccines. Vet Rec. 1982; 110:27–32.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of an inactivated rabies vaccine for animals derived from the recombinant ERAGS strain
- Adenovirus Vectors: Excellent Tools for Vaccine Development
- Antibody Response in Korean Raccoon Dogs Inoculated with Inactivated Rabies Vaccines
- Recharacterization of the Canine Adenovirus Type 1 Vaccine Strain based on the Biological and Molecular Properties
- Incidence of canine viral diseases and prevalence of virus neutralization antibodies of canine distemper virus, adenovirus type 2, parvovirus, and parainfluenza virus type 5 in Korean dogs