Yonsei Med J.
2020 Jan;61(1):85-93. 10.3349/ymj.2020.61.1.85.
Follistatin Mitigates Myofibroblast Differentiation and Collagen Synthesis of Fibroblasts from Scar Tissue around Injured Flexor Tendons
- Affiliations
-
- 1BK21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea. YRCHOI@yuhs.ac
- KMID: 2466340
- DOI: http://doi.org/10.3349/ymj.2020.61.1.85
Abstract
- PURPOSE
The aim of this study was to investigate the effect of FST gene on the inhibition of fibrosis in fibroblastic cells from scar tissue around repaired zone II flexor tendons.
MATERIALS AND METHODS
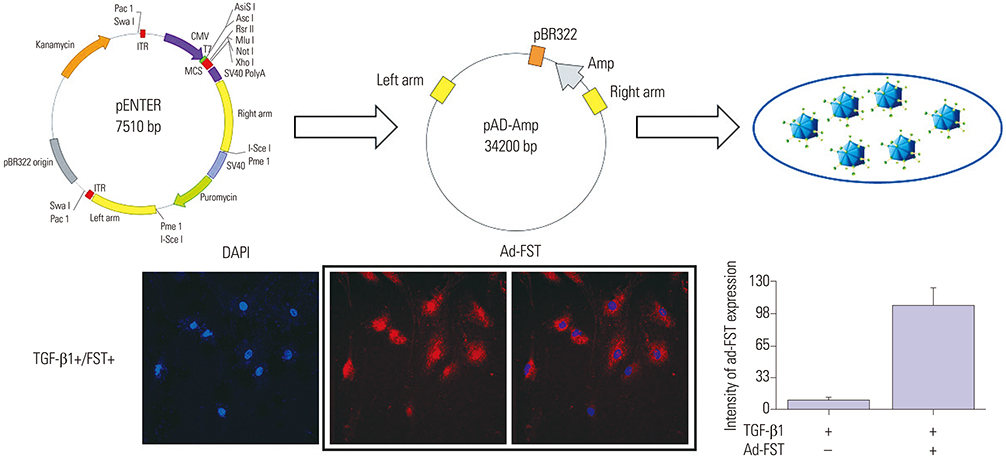
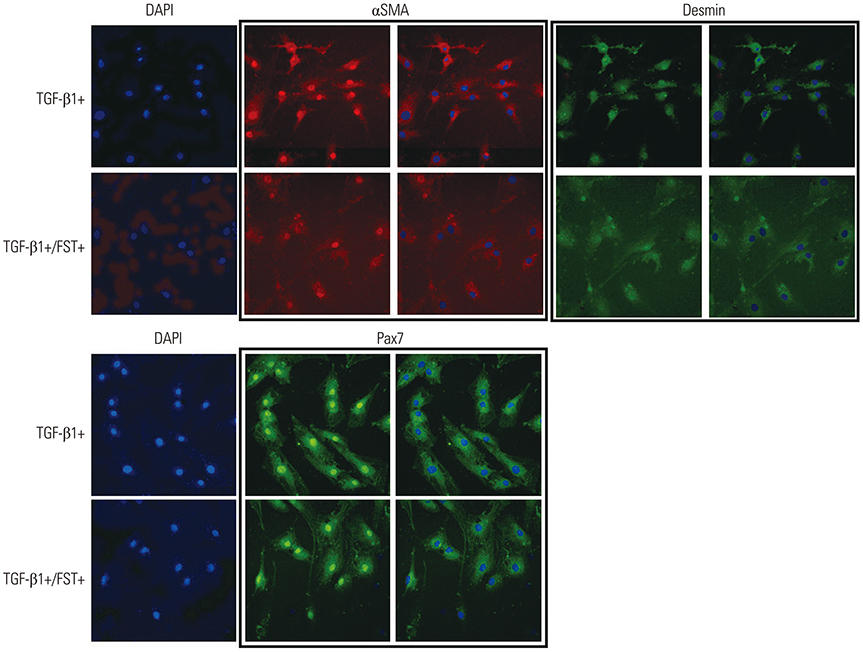
Immunohistochemistry was conducted on fibroblast cells transfected with adenovirus-LacZ (Ad-LacZ) as a marker gene (control), or with adenovirus-FST (Ad-FST) as a therapeutic gene. Fibroblast cultures without adenoviral exposure served as controls.
RESULTS
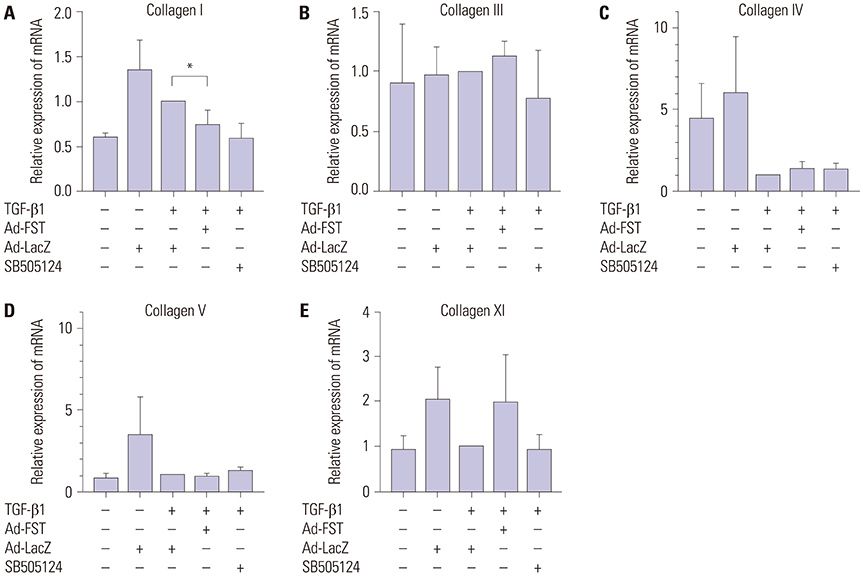
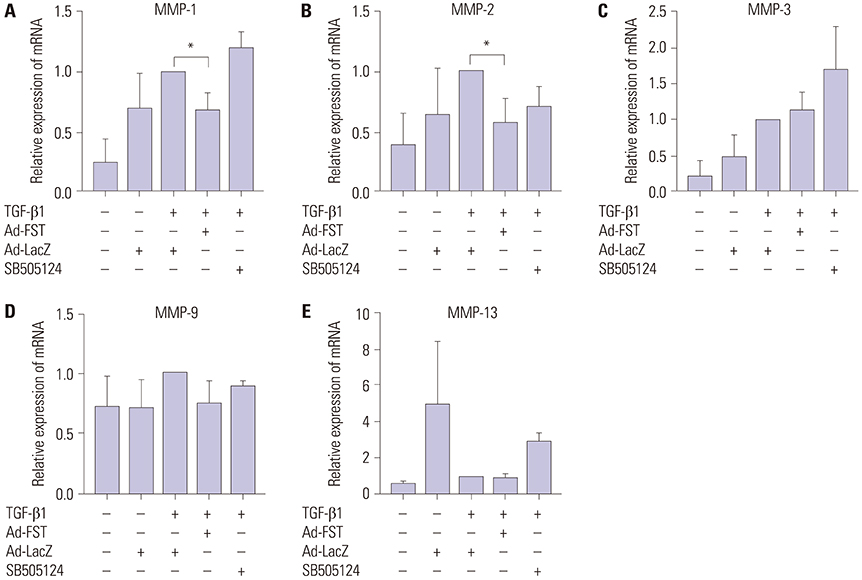
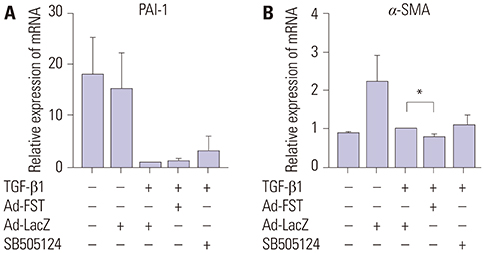
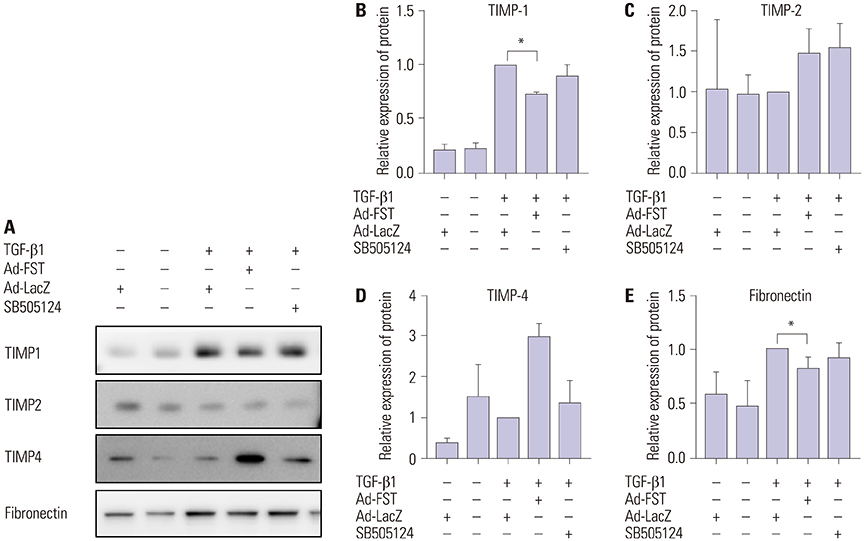
Fibroblastic cells transfected with Ad-FST demonstrated significant decrease in collagen type I, MMP-1, MMP2, and α-SMA mRNA expressions compared to those transfected with Ad-LacZ. In addition, fibroblastic cells transfected with Ad-FST exhibited significant decrease in MMP-1, TIMP-1, fibronectin, PAI-1, TRPV4, α-SMA, desmin, and PAX7 protein expressions.
CONCLUSION
Based on these findings, we conclude that FST may be a novel therapeutic strategy for preventing scar adhesions around repaired tendons by inhibiting fibroblasts from differentiating into myofibroblasts, in addition to producing type I collagen and regulating extracellular matrix turnover via the downregulation of MMP-1 and TIMP-1. FST may also decrease contracture of the scar by inhibiting Ca²âº-dependent cell contraction.
Keyword
MeSH Terms
-
Cicatrix*
Collagen Type I
Collagen*
Contracture
Desmin
Down-Regulation
Extracellular Matrix
Fibroblasts*
Fibronectins
Fibrosis
Follistatin*
Genetic Therapy
Immunohistochemistry
Myofibroblasts*
Plasminogen Activator Inhibitor 1
RNA, Messenger
Tendon Injuries
Tendons*
Tissue Inhibitor of Metalloproteinase-1
Collagen
Collagen Type I
Desmin
Fibronectins
Follistatin
Plasminogen Activator Inhibitor 1
RNA, Messenger
Tissue Inhibitor of Metalloproteinase-1
Figure
Reference
-
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008; 39:1338–1344.
Article2. Dy CJ, Daluiski A, Do HT, Hernandez-Soria A, Marx R, Lyman S. The epidemiology of reoperation after flexor tendon repair. J Hand Surg Am. 2012; 37:919–924.
Article3. Dy CJ, Hernandez-Soria A, Ma Y, Roberts TR, Daluiski A. Complications after flexor tendon repair: a systematic review and metaanalysis. J Hand Surg Am. 2012; 37:543–551.
Article4. Hill C, Riaz M, Mozzam A, Brennen MD. A regional audit of hand and wrist injuries. A study of 4873 injuries. J Hand Surg Br. 1998; 23:196–200.5. Tang JB. New developments are improving flexor tendon repair. Plast Reconstr Surg. 2018; 141:1427–1437.
Article6. Ketchum LD. Effects of triamcinolone on tendon healing and function. A laboratory study. Plast Reconstr Surg. 1971; 47:471–482.7. Kulick MI, Brazlow R, Smith S, Hentz VR. Injectable ibuprofen:preliminary evaluation of its ability to decrease peritendinous adhesions. Ann Plast Surg. 1984; 13:459–467.
Article8. Amiel D, Ishizue K, Billings E Jr, Wiig M, Vande Berg J, Akeson WH, et al. Hyaluronan in flexor tendon repair. J Hand Surg Am. 1989; 14:837–843.
Article9. Salti NI, Tuel RJ, Mass DP. Effect of hyaluronic acid on rabbit profundus flexor tendon healing in vitro. J Surg Res. 1993; 55:411–415.
Article10. Wiig M, Abrahamsson SO, Lundborg G. Tendon repair--cellular activities in rabbit deep flexor tendons and surrounding synovial sheaths and the effects of hyaluronan: an experimental study in vivo and in vitro. J Hand Surg Am. 1997; 22:818–825.
Article11. Nyska M, Porat S, Nyska A, Rousso M, Shoshan S. Decreased adhesion formation in flexor tendons by topical application of enriched collagen solution--a histological study. Arch Orthop Trauma Surg. 1987; 106:192–194.
Article12. Akali A, Khan U, Khaw PT, McGrouther AD. Decrease in adhesion formation by a single application of 5-fluorouracil after flexor tendon injury. Plast Reconstr Surg. 1999; 103:151–158.
Article13. Karaaltin MV, Ozalp B, Dadaci M, Kayikcioglu A, Kecik A, Oner F. The effects of 5-fluorouracil on flexor tendon healing by using a biodegradable gelatin, slow releasing system: experimental study in a hen model. J Hand Surg Eur Vol. 2013; 38:651–657.
Article14. Bartle BK, Telepun GM, Goldberg NH. Development of a synthetic replacement for flexor tendon pulleys using expanded polytetrafluoroethylene membrane. Ann Plast Surg. 1992; 28:266–270.
Article15. Golash A, Kay A, Warner JG, Peck F, Watson JS, Lees VC. Efficacy of ADCON-T/N after primary flexor tendon repair in Zone II: a controlled clinical trial. J Hand Surg Br. 2003; 28:113–115.
Article16. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012; 18:1028–1040.
Article17. Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000; 105:148–155.
Article18. Chen CH, Zhou YL, Wu YF, Cao Y, Gao JS, Tang JB. Effectiveness of microRNA in Down-regulation of TGF-beta gene expression in digital flexor tendons of chickens: in vitro and in vivo study. J Hand Surg Am. 2009; 34:1777–1784.19. Bates SJ, Morrow E, Zhang AY, Pham H, Longaker MT, Chang J. Mannose-6-phosphate, an inhibitor of transforming growth factor-beta, improves range of motion after flexor tendon repair. J Bone Joint Surg Am. 2006; 88:2465–2472.
Article20. Katzel EB, Wolenski M, Loiselle AE, Basile P, Flick LM, Langstein HN, et al. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res. 2011; 29:684–693.
Article21. Jørgensen HG, McLellan SD, Crossan JF, Curtis AS. Neutralisation of TGF beta or binding of VLA-4 to fibronectin prevents rat tendon adhesion following transection. Cytokine. 2005; 30:195–202.
Article22. Lees VC, Warwick D, Gillespie P, Brown A, Akhavani M, Dewer D, et al. A multicentre, randomized, double-blind trial of the safety and efficacy of mannose-6-phosphate in patients having Zone II flexor tendon repairs. J Hand Surg Eur Vol. 2015; 40:682–694.
Article23. Sugino K, Kurosawa N, Nakamura T, Takio K, Shimasaki S, Ling N, et al. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J Biol Chem. 1993; 268:15579–15587.
Article24. Hedger MP, Winnall WR, Phillips DJ, de Kretser DM. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm. 2011; 85:255–297.
Article25. Hashimoto O, Nakamura T, Shoji H, Shimasaki S, Hayashi Y, Sugino H. A novel role of follistatin, an activin-binding protein, in the inhibition of activin action in rat pituitary cells. Endocytotic degradation of activin and its acceleration by follistatin associated with cell-surface heparan sulfate. J Biol Chem. 1997; 272:13835–13842.
Article26. Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006; 290:G137–G144.
Article27. Aoki F, Kurabayashi M, Hasegawa Y, Kojima I. Attenuation of bleomycin-induced pulmonary fibrosis by follistatin. Am J Respir Crit Care Med. 2005; 172:713–720.
Article28. Forrester HB, Li J, Leong T, McKay MJ, Sprung CN. Identification of a radiation sensitivity gene expression profile in primary fibroblasts derived from patients who developed radiotherapy-induced fibrosis. Radiother Oncol. 2014; 111:186–193.
Article29. Koob TJ, Summers AP. Tendon--bridging the gap. Comp Biochem Physiol A Mol Integr Physiol. 2002; 133:905–909.
Article30. Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br Med Bull. 2009; 90:85–109.
Article31. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015; 33:832–839.
Article32. Wu YF, Mao WF, Zhou YL, Wang XT, Liu PY, Tang JB. Adeno-associated virus-2-mediated TGF-β1 microRNA transfection inhibits adhesion formation after digital flexor tendon injury. Gene Ther. 2016; 23:167–175.
Article33. DePaolo LV. Inhibins, activins, and follistatins: the saga continues. Proc Soc Exp Biol Med. 1997; 214:328–339.
Article34. Harrington AE, Morris-Triggs SA, Ruotolo BT, Robinson CV, Ohnuma S, Hyvönen M. Structural basis for the inhibition of activin signalling by follistatin. EMBO J. 2006; 25:1035–1045.
Article35. Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol. 2012; 180:12–16.
Article36. Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007; 127:526–537.
Article37. Sandbo N, Dulin N. Actin cytoskeleton in myofibroblast differentiation: ultrastructure defining form and driving function. Transl Res. 2011; 158:181–196.
Article38. Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010; 316:2390–2401.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Proliferation and the Collagen Synthesis of the Fibroblasts Cultured from Normal Skin and Hypertrophic Scar
- Effect of Palmitoyl-Pentapeptide (Pal-KTTKS) on Wound Contractile Process in Relation with Connective Tissue Growth Factor and α-Smooth Muscle Actin Expression
- A Morphological Study of Exposed Chicken Flexor Tendons
- Collagen Gel Contraction by Cultured Fibroblasts Derived from Normal Skin, Oral Mucosa, and Hypertrophic Scar
- Experimental Study on the Adhesion of the Flexor Tendon in Chickens