Cancer Res Treat.
2019 Apr;51(2):706-717. 10.4143/crt.2018.316.
The Clinical Value of PELP1 for Breast Cancer: A Comparison with Multiple Cancers and Analysis in Breast Cancer Subtypes
- Affiliations
-
- 1Department of Pathology, Peking University Shenzhen Hospital, Shenzhen, China.
- 2Department of Anatomical and Cellular Pathology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong. garytse@cuhk.edu.hk
- 3Department of Pathology, Kwong Wah Hospital, Hong Kong.
- 4Department of Pathology, Tuen Mun Hosiptal, Hong Kong.
- KMID: 2464416
- DOI: http://doi.org/10.4143/crt.2018.316
Abstract
- PURPOSE
Proline, glutamic acid, and leucine-rich protein 1 (PELP1), a novel nuclear receptor (NR) co-regulator, is highly expressed in breast cancer. We investigated its expression in breast cancer subtypes, in comparison with other breast markers as well as cancers from different sites. Its prognostic relevance with different subtypes and other NR expression was also examined in breast cancers.
MATERIALS AND METHODS
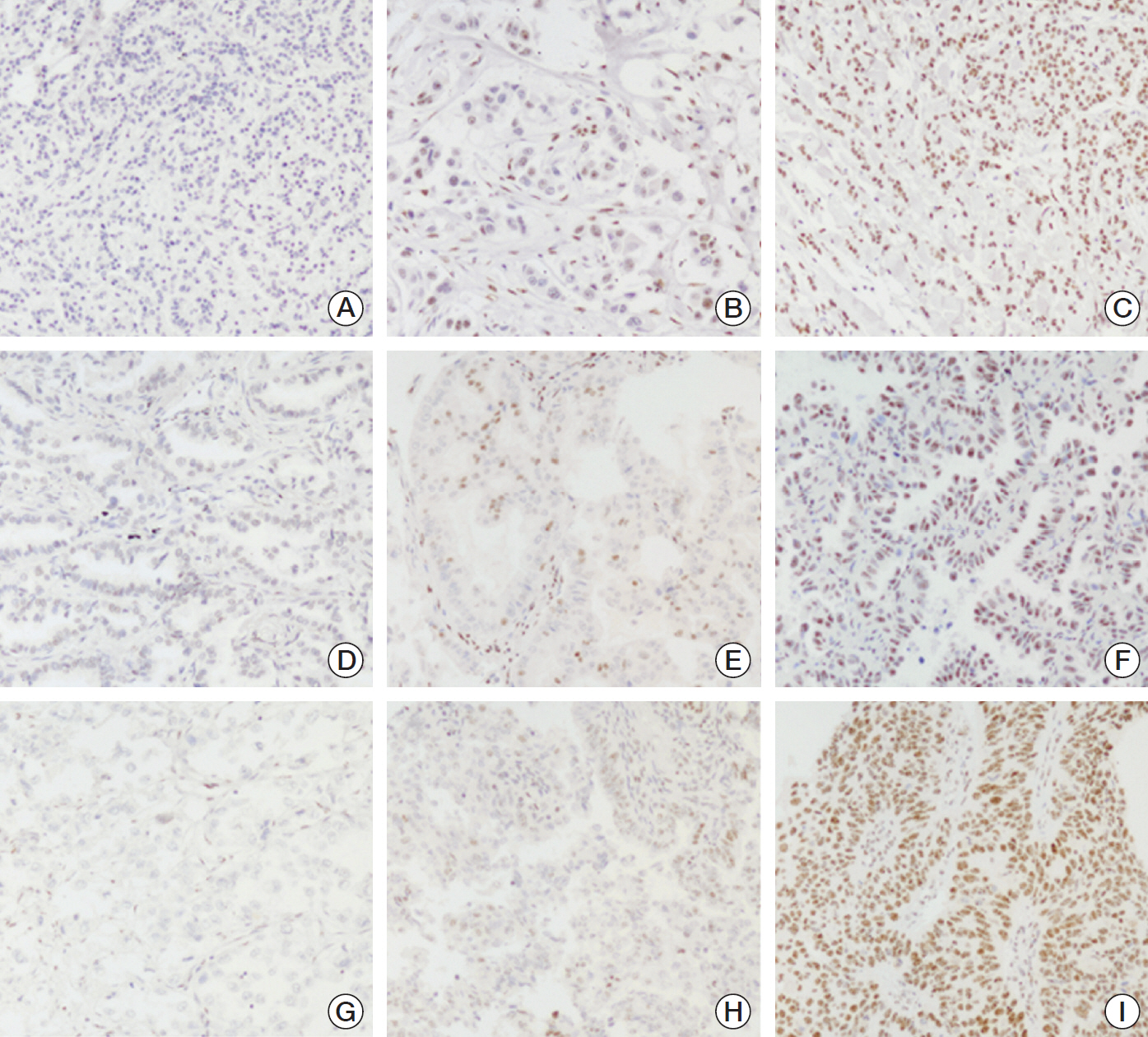
Immunohistochemical analysis was performed on totally 1,944 cancers from six different organs.
RESULTS
PELP1 expression rate was the highest in breast cancers (70.5%) among different cancers. Compared to GATA3, mammaglobin and gross cystic disease fluid protein 15, PELP1 was less sensitive than GATA3 for luminal cancers, but was the most sensitive for non-luminal cancers. PELP1 has low expression rate (<20%) in colorectal cancers, gastric cancers and renal cell carcinomas, but higher in lung cancers (49.1%) and ovarian cancers (42.3%). In breast cancer, PELP1 expression was an independent adverse prognostic factor for non-luminal cancers (disease-free survival [DFS]: hazard ratio [HR], 1.403; p=0.012 and breast cancer specific survival [BCSS]: HR, 1.443; p=0.015). Interestingly, its expression affected the prognostication of androgen receptor (AR). AR(pos)PELP1(lo) luminal cancer showed the best DFS (log-rank=8.563, p=0.036) while AR(neg)PELP1(hi) non-luminal cancers showed the worst DFS (log-rank=9.536, p=0.023).
CONCLUSION
PELP1 is a sensitive marker for breast cancer, particularly non-luminal cases. However, its considerable expression in lung and ovarian cancers may limit its utility in differential diagnosis in some scenarios. PELP1 expression was associated with poor outcome in non-luminal cancers and modified the prognostic effects of AR, suggesting the potential significance of NR co-regulator in prognostication.
MeSH Terms
Figure
Reference
-
References
1. Chakravarty D, Tekmal RR, Vadlamudi RK. PELP1: a novel therapeutic target for hormonal cancers. IUBMB Life. 2010; 62:162–9.
Article2. Girard BJ, Daniel AR, Lange CA, Ostrander JH. PELP1: a review of PELP1 interactions, signaling, and biology. Mol Cell Endocrinol. 2014; 382:642–51.
Article3. Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, et al. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005; 65:7724–32.
Article4. Mann M, Zou Y, Chen Y, Brann D, Vadlamudi R. PELP1 oncogenic functions involve alternative splicing via PRMT6. Mol Oncol. 2014; 8:389–400.
Article5. Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic potential of the nuclear receptor coregulator proline-, glutamic acid-, leucine-rich protein 1/modulator of the nongenomic actions of the estrogen receptor. Cancer Res. 2007; 67:5505–12.
Article6. Cortez V, Samayoa C, Zamora A, Martinez L, Tekmal RR, Vadlamudi RK. PELP1 overexpression in the mouse mammary gland results in the development of hyperplasia and carcinoma. Cancer Res. 2014; 74:7395–405.
Article7. Brann DW, Zhang QG, Wang RM, Mahesh VB, Vadlamudi RK. PELP1: a novel estrogen receptor-interacting protein. Mol Cell Endocrinol. 2008; 290:2–7.8. Kumar R, Zhang H, Holm C, Vadlamudi RK, Landberg G, Rayala SK. Extranuclear coactivator signaling confers insensitivity to tamoxifen. Clin Cancer Res. 2009; 15:4123–30.
Article9. Habashy HO, Powe DG, Rakha EA, Ball G, Macmillan RD, Green AR, et al. The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat. 2010; 120:603–12.
Article10. Zhang Y, Dai J, McNamara KM, Bai B, Shi M, Chan MS, et al. Prognostic significance of proline, glutamic acid, leucine rich protein 1 (PELP1) in triple-negative breast cancer: a retrospective study on 129 cases. BMC Cancer. 2015; 15:699.
Article11. Dang DN, Raj G, Sarode V, Molberg KH, Vadlamudi RK, Peng Y. Significantly increased PELP1 protein expression in primary and metastatic triple-negative breast carcinoma: comparison with GATA3 expression and PELP1's potential role in triple-negative breast carcinoma. Hum Pathol. 2015; 46:1829–35.
Article12. Vadlamudi RK, Balasenthil S, Broaddus RR, Gustafsson JA, Kumar R. Deregulation of estrogen receptor coactivator proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor in human endometrial tumors. J Clin Endocrinol Metab. 2004; 89:6130–8.
Article13. Aust S, Horak P, Pils D, Pils S, Grimm C, Horvat R, et al. The prognostic value of estrogen receptor beta and proline-, glutamic acid- and leucine-rich protein 1 (PELP1) expression in ovarian cancer. BMC Cancer. 2013; 13:115.
Article14. Nair SS, Guo Z, Mueller JM, Koochekpour S, Qiu Y, Tekmal RR, et al. Proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor enhances androgen receptor functions through LIM-only coactivator, four-and-a-half LIM-only protein 2. Mol Endocrinol. 2007; 21:613–24.
Article15. Slowikowski BK, Galecki B, Dyszkiewicz W, Jagodzinski PP. Increased expression of proline-, glutamic acid- and leucine-rich protein PELP1 in non-small cell lung cancer. Biomed Pharmacother. 2015; 73:97–101.16. Ning Z, Zhang Y, Chen H, Wu J, Song T, Wu Q, et al. PELP1 suppression inhibits colorectal cancer through c-Src downregulation. Oxid Med Cell Longev. 2014; 2014:193523.
Article17. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–10.
Article18. Lakhani SR, Ellis IO, Schnitee SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press;2012.19. Tsang JY, Au WL, Lo KY, Ni YB, Hlaing T, Hu J, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat. 2017; 162:19–30.20. Tsang JY, Ni YB, Chan SK, Shao MM, Law BK, Tan PH, et al. Androgen receptor expression shows distinctive significance in ER positive and negative breast cancers. Ann Surg Oncol. 2014; 21:2218–28.
Article21. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24:2206–23.22. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004; 10:5367–74.
Article23. Dimple C, Nair SS, Rajhans R, Pitcheswara PR, Liu J, Balasenthil S, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008; 68:4902–9.
Article24. Yao T, Wang Q, Zhang W, Bian A, Zhang J. Identification of genes associated with renal cell carcinoma using gene expression profiling analysis. Oncol Lett. 2016; 12:73–8.
Article25. Grivas PD, Tzelepi V, Sotiropoulou-Bonikou G, Kefalopoulou Z, Papavassiliou AG, Kalofonos H. Expression of ERalpha, ERbeta and co-regulator PELP1/MNAR in colorectal cancer: prognostic significance and clinicopathologic correlations. Cell Oncol. 2009; 31:235–47.26. Nishimura R, Osako T, Nishiyama Y, Tashima R, Nakano M, Fujisue M, et al. Evaluation of factors related to late recurrence‒later than 10 years after the initial treatment‒in primary breast cancer. Oncology. 2013; 85:100–10.27. Tang D, Xu S, Zhang Q, Zhao W. The expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancer. Med Oncol. 2012; 29:526–33.
Article28. Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009; 69:6131–40.29. Yang L, Ravindranathan P, Ramanan M, Kapur P, Hammes SR, Hsieh JT, et al. Central role for PELP1 in nonandrogenic activation of the androgen receptor in prostate cancer. Mol Endocrinol. 2012; 26:550–61.
Article30. Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004; 64:6416–23.
Article31. Roy S, Chakravarty D, Cortez V, De Mukhopadhyay K, Bandyopadhyay A, Ahn JM, et al. Significance of PELP1 in ER-negative breast cancer metastasis. Mol Cancer Res. 2012; 10:25–33.
Article32. Rajhans R, Nair HB, Nair SS, Cortez V, Ikuko K, Kirma NB, et al. Modulation of in situ estrogen synthesis by proline-, glutamic acid-, and leucine-rich protein-1: potential estrogen receptor autocrine signaling loop in breast cancer cells. Mol Endocrinol. 2008; 22:649–64.
Article33. Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007; 5:e004.
Article34. Sareddy GR, Vadlamudi RK. PELP1: Structure, biological function and clinical significance. Gene. 2016; 585:128–34.
Article35. Manavathi B, Nair SS, Wang RA, Kumar R, Vadlamudi RK. Proline-, glutamic acid-, and leucine-rich protein-1 is essential in growth factor regulation of signal transducers and activators of transcription 3 activation. Cancer Res. 2005; 65:5571–7.
Article36. Truong TH, Hu H, Temiz NA, Hagen KM, Girard BJ, Brady NJ, et al. Cancer stem cell phenotypes in ER(+) breast cancer models are promoted by PELP1/AIB1 complexes. Mol Cancer Res. 2018; 16:707–19.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Highligts of 28th Annual Meeting of San Antonio Breast Cancer Symposium
- Bilateral breast carcinoma
- Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosisâ€
- Surgery of Breast Cancer during the Last 5 Years: More Sophisticated and Specialized?
- Misdiagnosed Breast Cancer on Mammography Retrospective Analysis in 17 Cases