Allergy Asthma Immunol Res.
2020 Jan;12(1):110-124. 10.4168/aair.2020.12.1.110.
Reversal of Olfactory Disturbance in Allergic Rhinitis Related to OMP Suppression by Intranasal Budesonide Treatment
- Affiliations
-
- 1Department of Otorhinolaryngology, Inha University School of Medicine, Incheon, Korea. inhaorl@inha.ac.kr
- KMID: 2462576
- DOI: http://doi.org/10.4168/aair.2020.12.1.110
Abstract
- PURPOSE
We evaluated the severity of olfactory disturbance (OD) in the murine model of allergic rhinitis (AR) and local allergic rhinitis (LAR) in mice. We also investigated the therapeutic effect of an intranasal steroid on OD.
METHODS
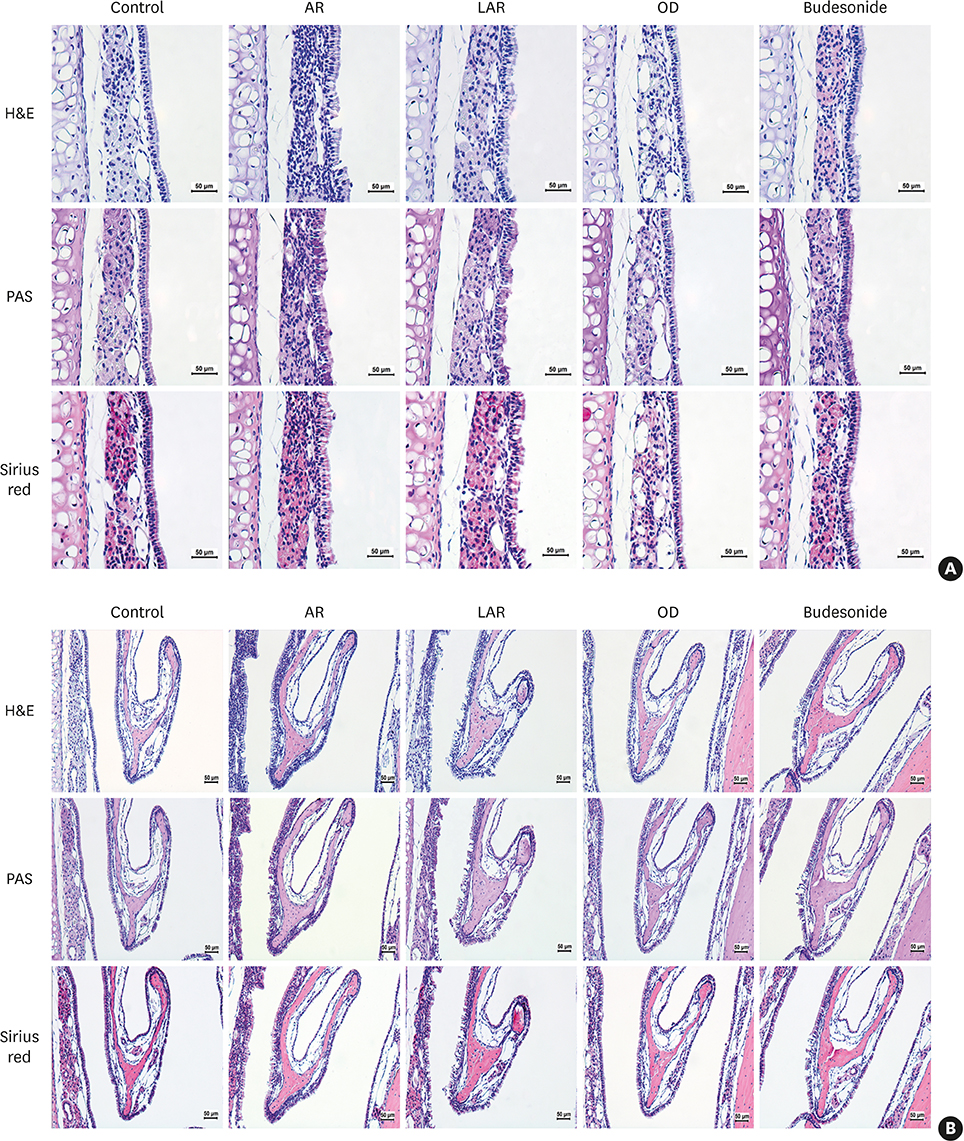
Forty BALB/c mice were divided into 5 groups (n = 8 for each). The control group was sensitized intraperitoneally (i.p.) and challenged intranasally (i.n.) with saline. Mice in the AR group got i.p. and i.n. ovalbumin (OVA) administration for AR induction. The LAR group was challenged i.n. with 1% OVA for inducing local nasal allergic inflammation, without inducing the systemic allergy. The OD group got an i.p. methimazole administration (75 mg/kg) to induce total destruction of olfactory mucosa. Mice in the intranasal budesonide group received i.n. budesonide (12.8 μg per time, 30 minutes after the i.n. OVA challenge) while using OVA to cause systemic allergies. We conducted a buried-food pellet test to functionally assess the degree of OD in each group by measuring the time taken until finding hidden food. We evaluated the damage to olfactory epithelium using histopathologic evaluation and compared the degree of olfactory marker protein (OMP) expression in olfactory epithelium using immunofluorescent staining.
RESULTS
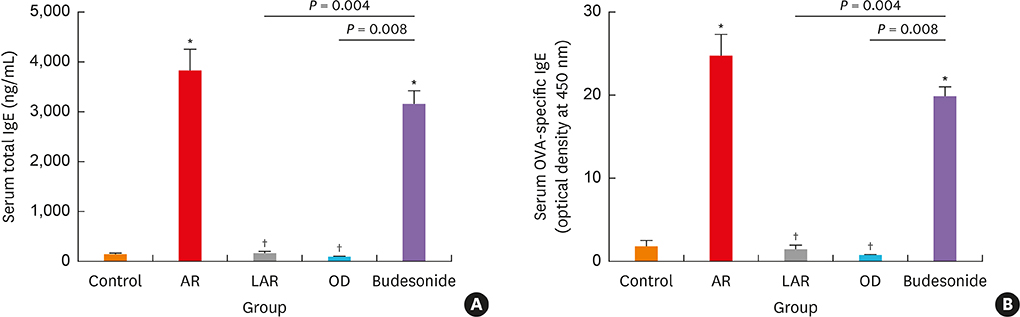
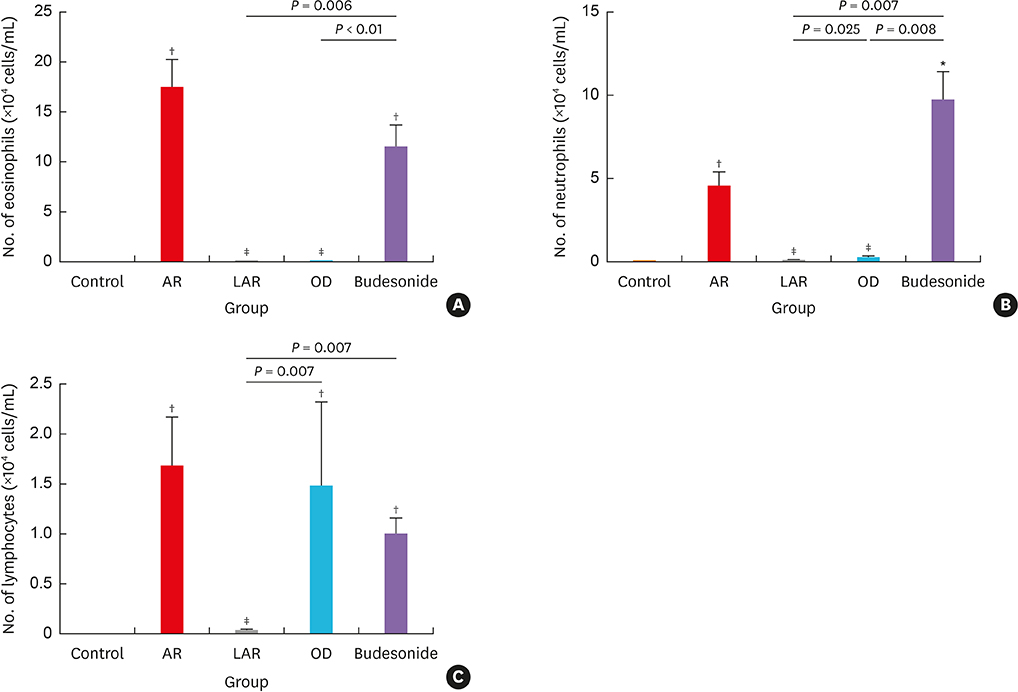
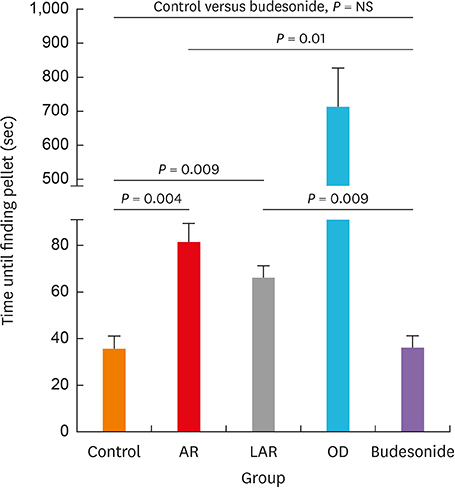
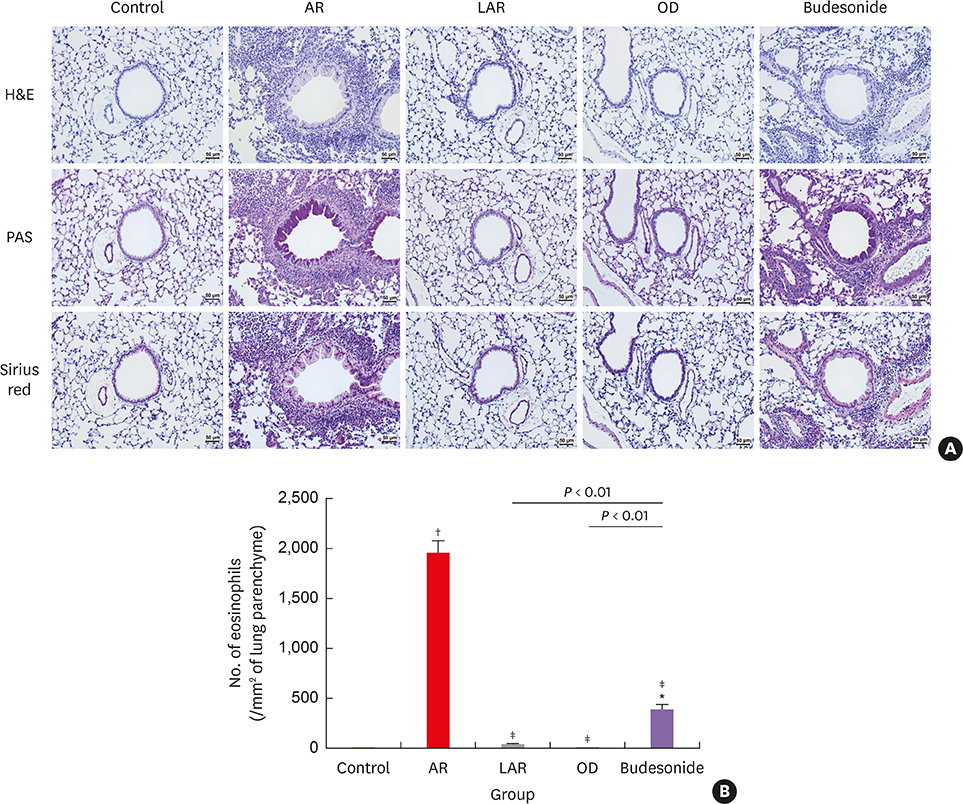
Mice of the AR (81.3 ± 19.8 seconds) and LAR groups (66.2 ± 12.7 seconds) spent significantly more time to detect the pellets than the control group (35.6 ± 12.2 seconds, P < 0.01). After treatment, the intranasal budesonide group exhibited significantly better results (35.8 ± 11.9 seconds) compared with the AR and LAR groups (P < 0.01). The AR and LAR groups showed considerable olfactory epithelial damage and suppression of OMP expression compared with the control group. In the intranasal budesonide group, the olfactory lesions and OMP expression had improved substantially.
CONCLUSIONS
OD may be caused by olfactory epithelial damage and suppression of OMP expression in nasal allergic inflammation and could be reversed using an intranasal steroid.
Keyword
MeSH Terms
Figure
Reference
-
1. Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017; 140:950–958.2. Cowart BJ, Flynn-Rodden K, McGeady SJ, Lowry LD. Hyposmia in allergic rhinitis. J Allergy Clin Immunol. 1993; 91:747–751.
Article3. Binder E, Holopainen E, Malmberg H, Salo O. Anamnestic data in allergic rhinitis. Allergy. 1982; 37:389–396.
Article4. Chaiyasate S, Roongrotwattanasiri K, Fooanant S, Sumitsawan Y. Key nasal symptoms predicting a positive skin test in allergic rhinitis and patient characteristics according to ARIA classification. J Med Assoc Thai. 2009; 92:377–381.5. Di Lorenzo G, Pacor ML, Amodio E, Leto-Barone MS, La Piana S, D'Alcamo A, et al. Differences and similarities between allergic and nonallergic rhinitis in a large sample of adult patients with rhinitis symptoms. Int Arch Allergy Immunol. 2011; 155:263–270.
Article6. Stuck BA, Hummel T. Olfaction in allergic rhinitis: a systematic review. J Allergy Clin Immunol. 2015; 136:1460–1470.7. Church JA, Bauer H, Bellanti JA, Satterly RA, Henkin RI. Hyposmia associated with atopy. Ann Allergy. 1978; 40:105–109.8. Klimek L, Eggers G. Olfactory dysfunction in allergic rhinitis is related to nasal eosinophilic inflammation. J Allergy Clin Immunol. 1997; 100:158–164.
Article9. Becker S, Pflugbeil C, Gröger M, Canis M, Ledderose GJ, Kramer MF. Olfactory dysfunction in seasonal and perennial allergic rhinitis. Acta Otolaryngol. 2012; 132:763–768.
Article10. Gómez F, Rondón C, Salas M, Campo P. Local allergic rhinitis: mechanisms, diagnosis and relevance for occupational rhinitis. Curr Opin Allergy Clin Immunol. 2015; 15:111–116.11. Campo P, Rondón C, Gould HJ, Barrionuevo E, Gevaert P, Blanca M. Local IgE in non-allergic rhinitis. Clin Exp Allergy. 2015; 45:872–881.
Article12. Rondón C, Campo P, Togias A, Fokkens WJ, Durham SR, Powe DG, et al. Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol. 2012; 129:1460–1467.
Article13. Chang GU, Jang TY, Kim KS, Choi H, Kim YH. Nonspecific hyper-reactivity and localized allergy: cause of discrepancy between skin prick and nasal provocation test. Otolaryngol Head Neck Surg. 2014; 150:194–200.14. Chen BW, Qu SH, Li M, Ye LS, Zhang SJ, Qin TJ, et al. A murine model of local allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016; 51:533–537.15. Kato Y, Akasaki S, Muto-Haenuki Y, Fujieda S, Matsushita K, Yoshimoto T. Nasal sensitization with ragweed pollen induces local-allergic-rhinitis-like symptoms in mice. PLoS One. 2014; 9:e103540.
Article16. Jang TY, Park CS, Kim KS, Heo MJ, Kim YH. Benzaldehyde suppresses murine allergic asthma and rhinitis. Int Immunopharmacol. 2014; 22:444–450.
Article17. Jang TY, Jung AY, Kim YH. Hormetic effect of chronic hypergravity in a mouse model of allergic asthma and rhinitis. Sci Rep. 2016; 6:27260.
Article18. Jang TY, Jung AY, Kyung TS, Kim DY, Hwang JH, Kim YH. Anti-allergic effect of luteolin in mice with allergic asthma and rhinitis. Cent Eur J Immunol. 2017; 42:24–29.
Article19. Jung AY, Heo MJ, Kim YH. Glucosamine has an antiallergic effect in mice with allergic asthma and rhinitis. Int Forum Allergy Rhinol. 2017; 7:763–769.
Article20. Ogawa T, Takezawa K, Shimizu S, Shimizu T. Valproic acid promotes neural regeneration of olfactory epithelium in adult mice after methimazole-induced damage. Am J Rhinol Allergy. 2014; 28:e95–9.
Article21. Zhou M, Du D, Zhao K, Zheng C. In vivo intranasal anti-CD23 treatment inhibits allergic responses in a murine model of allergic rhinitis. J Mol Histol. 2013; 44:327–338.22. Lehmkuhl AM, Dirr ER, Fleming SM. Olfactory assays for mouse models of neurodegenerative disease. J Vis Exp. 2014; 25:e51804.
Article23. Guss J, Doghramji L, Reger C, Chiu AG. Olfactory dysfunction in allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2009; 71:268–272.
Article24. Apter AJ, Mott AE, Frank ME, Clive JM. Allergic rhinitis and olfactory loss. Ann Allergy Asthma Immunol. 1995; 75:311–316.25. Apter AJ, Gent JF, Frank ME. Fluctuating olfactory sensitivity and distorted odor perception in allergic rhinitis. Arch Otolaryngol Head Neck Surg. 1999; 125:1005–1010.
Article26. Catana IV, Chirila M, Negoias S, Bologa R, Cosgarea M. Effects of corticosteroids on hyposmia in persistent allergic rhinitis. Clujul Med. 2013; 86:117–120.27. Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. 2000; 110:1071–1077.28. Rhee CS, Wee JH, Ahn JC, Lee WH, Tan KL, Ahn S, et al. Prevalence, risk factors and comorbidities of allergic rhinitis in South Korea: the Fifth Korea National Health and Nutrition Examination Survey. Am J Rhinol Allergy. 2014; 28:e107–e114.
Article29. Moll B, Klimek L, Eggers G, Mann W. Comparison of olfactory function in patients with seasonal and perennial allergic rhinitis. Allergy. 1998; 53:297–301.
Article30. Rydzewski B, Pruszewicz A, Sulkowski WJ. Assessment of smell and taste in patients with allergic rhinitis. Acta Otolaryngol. 2000; 120:323–326.31. Fong KJ, Kern RC, Foster JD, Zhao JC, Pitovski DZ. Olfactory secretion and sodium, potassium-adenosine triphosphatase: regulation by corticosteroids. Laryngoscope. 1999; 109:383–388.
Article32. Epstein VA, Bryce PJ, Conley DB, Kern RC, Robinson AM. Intranasal Aspergillus fumigatus exposure induces eosinophilic inflammation and olfactory sensory neuron cell death in mice. Otolaryngol Head Neck Surg. 2008; 138:334–339.
Article33. Kim YH, Park CS, Jang TY. Immunologic properties and clinical features of local allergic rhinitis. J Otolaryngol Head Neck Surg. 2012; 41:51–57.34. Kim YH, Jang TY. Clinical characteristics and therapeutic outcomes of patients with localized mucosal allergy. Am J Rhinol Allergy. 2010; 24:e89–92.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Comparison of the Therapeutic Effects of Powder and Aerosolized Budesonide in the Treatment of Perennial Allergic Rhinitis
- A Case of Allergic Contact Dermatitis to Budesonide in a Nasal Spray
- A comparative study of intranasal budesonide and oral terfenadine in perennial allergic rhinitics: effect on the symptom score and nasal secretion eosinophils
- Immunohistological Study of the Effects of Intranasal ZnSO4 Instillation on the Mouse Olfactory Epithelium and Olfactory Bulb
- The Effect of Topical Steroid Nasal Instillation in Induced Anosmic Mice