J Korean Med Assoc.
2019 Nov;62(11):577-585. 10.5124/jkma.2019.62.11.577.
Diagnosis and treatment of adult Moyamoya disease
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea. eunkim@snu.ac.kr
- KMID: 2462241
- DOI: http://doi.org/10.5124/jkma.2019.62.11.577
Abstract
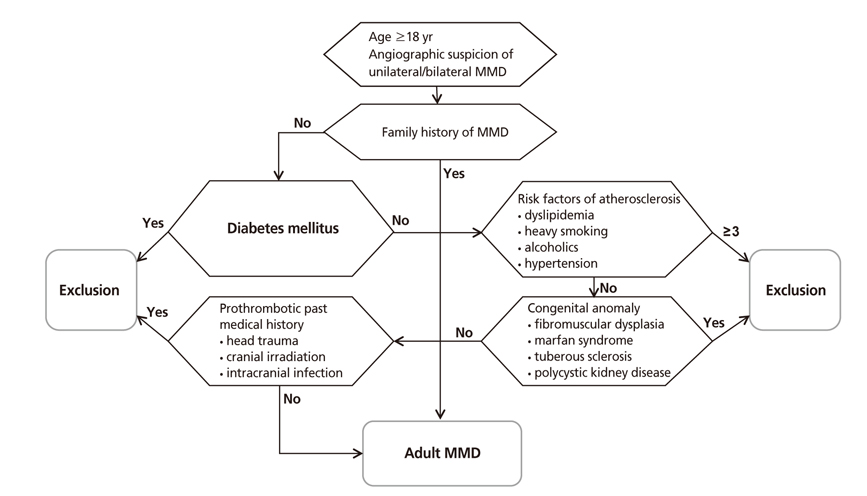
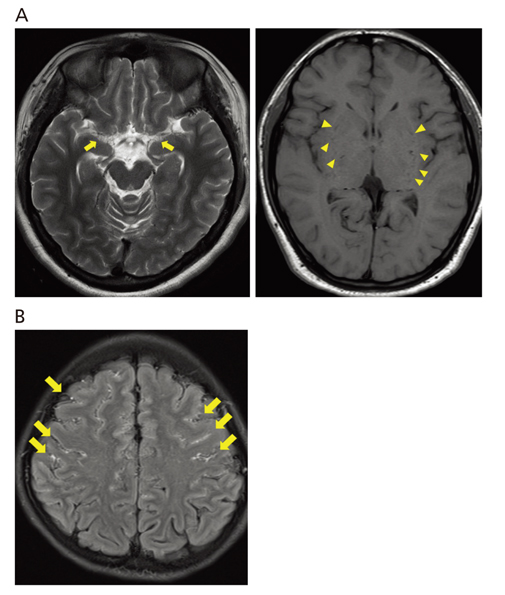
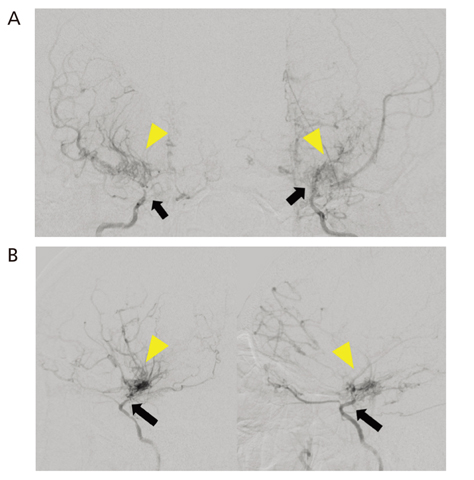
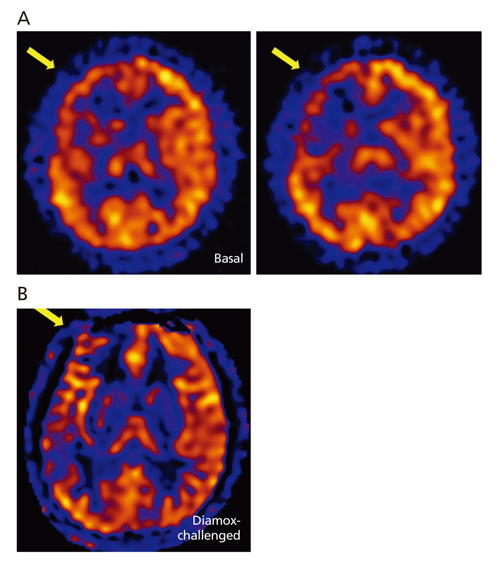
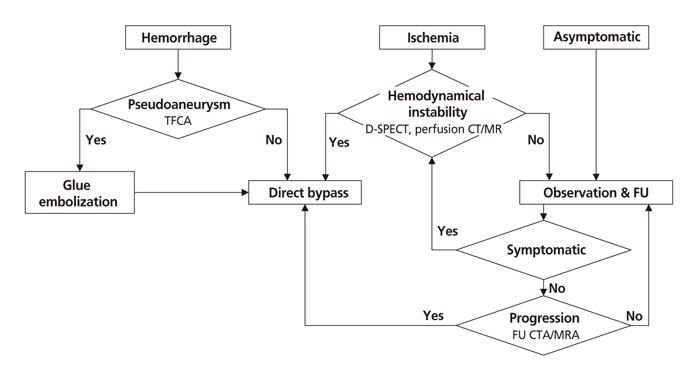
- Moyamoya disease (MMD) refers to a chronic progressive steno-occlusive disease at the distal portion of the internal carotid artery with abnormal collateral vessel formation of unknown etiology. The definite diagnosis of MMD requires cerebral angiography or magnetic resonance angiography and/or magnetic resonance imaging after excluding other underlying diseases, particularly in adult patients. The treatment aims to improve regional cerebral blood flow to prevent cerebral ischemic events and alleviate hemodynamic instability that can provoke cerebral hemorrhage. Although various surgical revascularization methods have been introduced, combined revascularization surgery including direct revascularization is preferred over indirect revascularization only in adult MMD patients. Several recent studies have shown that surgical treatment has better outcomes and prognosis for symptomatic hemodynamically unstable MMD patients with both ischemic and hemorrhagic presentations. For asymptomatic patients, follow up with appropriate imaging is recommended. Surgery should be considered when new symptoms emerge with hemodynamic aggravation.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Moyamoya disease: insights into the clinical implications of the
RNF213 gene
Taedong Ok
Cardiovasc Prev Pharmacother. 2024;6(4):109-115. doi: 10.36011/cpp.2024.6.e14.
Reference
-
1. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969; 20:288–299.2. Ahn IM, Park DH, Hann HJ, Kim KH, Kim HJ, Ahn HS. Incidence, prevalence, and survival of moyamoya disease in Korea: a nationwide, population-based study. Stroke. 2014; 45:1090–1095.
Article3. Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008; 79:900–904.
Article4. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009; 360:1226–1237.
Article5. Kim JE, Kim KM, Kim JG, Kang HS, Bang JS, Son YJ, Han MH, Oh CW. Clinical features of adult moyamoya disease with special reference to the diagnosis. Neurol Med Chir (Tokyo). 2012; 52:311–317.
Article6. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997; 99 Suppl 2:S238–S240.7. Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, Hashikata H, Matsuura N, Yamazaki S, Toyoda A, Kikuta K, Takagi Y, Harada KH, Fujiyama A, Herzig R, Krischek B, Zou L, Kim JE, Kitakaze M, Miyamoto S, Nagata K, Hashimoto N, Koizumi A. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011; 6:e22542.
Article8. Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I, Tsurusaki Y, Doi H, Sakai H, Saitsu H, Shimojima K, Yamamoto T, Higurashi M, Kawahara N, Kawauchi H, Nagasaka K, Okamoto N, Mori T, Koyano S, Kuroiwa Y, Taguri M, Morita S, Matsubara Y, Kure S, Matsumoto N. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology. 2012; 78:803–810.
Article9. Mikami T, Suzuki H, Komatsu K, Mikuni N. Influence of inflammatory disease on the pathophysiology of moyamoya disease and quasi-moyamoya disease. Neurol Med Chir (Tokyo). 2019; 07. 06. DOI: 10.2176/nmc.ra.2019-0059. [Epub].
Article10. Bang OY, Chung JW, Cha J, Lee MJ, Yeon JY, Ki CS, Jeon P, Kim JS, Hong SC. A polymorphism in RNF213 is a susceptibility gene for intracranial atherosclerosis. PLoS One. 2016; 11:e0156607.
Article11. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis. Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo). 2012; 52:245–266.12. Mineharu Y, Takenaka K, Yamakawa H, Inoue K, Ikeda H, Kikuta KI, Takagi Y, Nozaki K, Hashimoto N, Koizumi A. Inheritance pattern of familial moyamoya disease: autosomal dominant mode and genomic imprinting. J Neurol Neurosurg Psychiatry. 2006; 77:1025–1029.
Article13. Ikezaki K, Inamura T, Kawano T, Fukui M. Clinical features of probable moyamoya disease in Japan. Clin Neurol Neurosurg. 1997; 99 Suppl 2:S173–S177.
Article14. Hayashi K, Suyama K, Nagata I. Clinical features of unilateral moyamoya disease. Neurol Med Chir (Tokyo). 2010; 50:378–385.
Article15. Maeda M, Tsuchida C. “Ivy sign” on fluid-attenuated inversion-recovery images in childhood moyamoya disease. AJNR Am J Neuroradiol. 1999; 20:1836–1838.16. Mossa-Basha M, de Havenon A, Becker KJ, Hallam DK, Levitt MR, Cohen WA, Hippe DS, Alexander MD, Tirschwell DL, Hatsukami T, Amlie-Lefond C, Yuan C. Added value of vessel wall magnetic resonance imaging in the differentiation of moyamoya vasculopathies in a non-asian cohort. Stroke. 2016; 47:1782–1788.
Article17. Kim JE, Jeon JS. An update on the diagnosis and treatment of adult moyamoya disease taking into consideration controversial issues. Neurol Res. 2014; 36:407–416.
Article18. Lee S, Yun TJ, Yoo RE, Yoon BW, Kang KM, Choi SH, Kim JH, Kim JE, Sohn CH, Han MH. Monitoring cerebral perfusion changes after revascularization in patients with moyamoya disease by using arterial spin-labeling MR imaging. Radiology. 2018; 288:565–572.
Article19. Lee M, Zaharchuk G, Guzman R, Achrol A, Bell-Stephens T, Steinberg GK. Quantitative hemodynamic studies in moyamoya disease: a review. Neurosurg Focus. 2009; 26:E5.20. Cho WS, Chung YS, Kim JE, Jeon JP, Son YJ, Bang JS, Kang HS, Sohn CH, Oh CW. The natural clinical course of hemodynamically stable adult moyamoya disease. J Neurosurg. 2015; 122:82–89.
Article21. Kuroda S, Ishikawa T, Houkin K, Nanba R, Hokari M, Iwasaki Y. Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. 2005; 36:2148–2153.
Article22. Lee SC, Jeon JS, Kim JE, Chung YS, Ahn JH, Cho WS, Son YJ, Bang JS, Kang HS, Oh CW. Contralateral progression and its risk factor in surgically treated unilateral adult moyamoya disease with a review of pertinent literature. Acta Neurochir (Wien). 2014; 156:103–111.
Article23. Cho WS, Kim JE, Kim CH, Ban SP, Kang HS, Son YJ, Bang JS, Sohn CH, Paeng JC, Oh CW. Long-term outcomes after combined revascularization surgery in adult moyamoya disease. Stroke. 2014; 45:3025–3031.
Article24. Kim T, Oh CW, Kwon OK, Hwang G, Kim JE, Kang HS, Cho WS, Bang JS. Stroke prevention by direct revascularization for patients with adult-onset moyamoya disease presenting with ischemia. J Neurosurg. 2016; 124:1788–1793.
Article25. Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, Nakagawara J, Takahashi JC. JAM Trial Investigators. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke. 2014; 45:1415–1421.
Article26. Mizoi K, Kayama T, Yoshimoto T, Nagamine Y. Indirect revascularization for moyamoya disease: is there a beneficial effect for adult patients? Surg Neurol. 1996; 45:541–548.
Article27. Jeon JP, Kim JE, Cho WS, Bang JS, Son YJ, Oh CW. Meta-analysis of the surgical outcomes of symptomatic moyamoya disease in adults. J Neurosurg. 2018; 128:793–799.
Article28. Kim T, Lee H, Bang JS, Kwon OK, Hwang G, Oh CW. Epidemiology of moyamoya disease in Korea: based on National Health Insurance Service data. J Korean Neurosurg Soc. 2015; 57:390–395.
Article29. Jang DK, Lee KS, Rha HK, Huh PW, Yang JH, Park IS, Ahn JG, Sung JH, Han YM. Bypass surgery versus medical treatment for symptomatic moyamoya disease in adults. J Neurosurg. 2017; 127:492–502.
Article30. Noh HJ, Kim SJ, Kim JS, Hong SC, Kim KH, Jun P, Bang OY, Chung CS, Lee KH, Lee KH, Kim GM. Long term outcome and predictors of ischemic stroke recurrence in adult moyamoya disease. J Neurol Sci. 2015; 359:381–388.
Article31. Kim KM, Kim JE, Cho WS, Kang HS, Son YJ, Han MH, Oh CW. Natural history and risk factor of recurrent hemorrhage in hemorrhagic adult moyamoya disease. Neurosurgery. 2017; 81:289–296.
Article32. Guzman R, Lee M, Achrol A, Bell-Stephens T, Kelly M, Do HM, Marks MP, Steinberg GK. Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg. 2009; 111:927–935.
Article33. Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK. Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis. 2008; 25:580–586.
Article34. Fujimura M, Kaneta T, Mugikura S, Shimizu H, Tominaga T. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol. 2007; 67:273–282.
Article35. Hwang JW, Yang HM, Lee H, Lee HK, Jeon YT, Kim JE, Lim YJ, Park HP. Predictive factors of symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in adult patients with moyamoya disease. Br J Anaesth. 2013; 110:773–779.
Article36. Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke. 2012; 43:2610–2616.
Article37. Heros RC, Scott RM, Kistler JP, Ackerman RH, Conner ES. Temporary neurological deterioration after extracranial-intracranial bypass. Neurosurgery. 1984; 15:178–185.
Article38. Cho WS, Lee HY, Kang HS, Kim JE, Bang JS, Oh CW. Symptomatic cerebral hyperperfusion on SPECT after indirect revascularization surgery for Moyamoya disease. Clin Nucl Med. 2013; 38:44–46.
Article39. Fujimura M, Niizuma K, Inoue T, Sato K, Endo H, Shimizu H, Tominaga T. Minocycline prevents focal neurological deterioration due to cerebral hyperperfusion after extracranialintracranial bypass for moyamoya disease. Neurosurgery. 2014; 74:163–170.
Article