Cancer Res Treat.
2019 Oct;51(4):1518-1526. 10.4143/crt.2019.008.
Loss of LKB1 Protein Expression Correlates with Increased Risk of Recurrence and Death in Patients with Resected, Stage II or III Colon Cancer
- Affiliations
-
- 1Laboratory of Translational Oncology, School of Medicine, University of Crete, Heraklion, Greece. oncsec@med.uoc.gr
- 2Department of Pathology, University General Hospital of Heraklion, Iraklio, Greece.
- 3Department of Surgical Oncology, University General Hospital of Heraklion, Iraklio, Greece.
- 4Department of Medical Oncology, University General Hospital of Heraklion, Iraklio, Greece.
- 5School of Medicine, University of Crete, Heraklion, Greece.
- KMID: 2460600
- DOI: http://doi.org/10.4143/crt.2019.008
Abstract
- PURPOSE
The purpose of this study was to investigate the prognostic significance of liver kinase b1 (LKB1) loss in patients with operable colon cancer (CC).
MATERIALS AND METHODS
Two hundred sixty-two specimens from consecutive patients with stage III or high-risk stage II CC, who underwent surgical resection with curative intent and received adjuvant chemotherapy with fluoropyrimidine and oxaliplatin, were analyzed for LKB1 protein expression loss, by immunohistochemistry as well as for KRAS exon 2 and BRAF(V600E) mutations by Sanger sequencing and TS, ERCC1, MYC, and NEDD9 mRNA expression by real-time quantitative reverse transcription polymerase chain reaction.
RESULTS
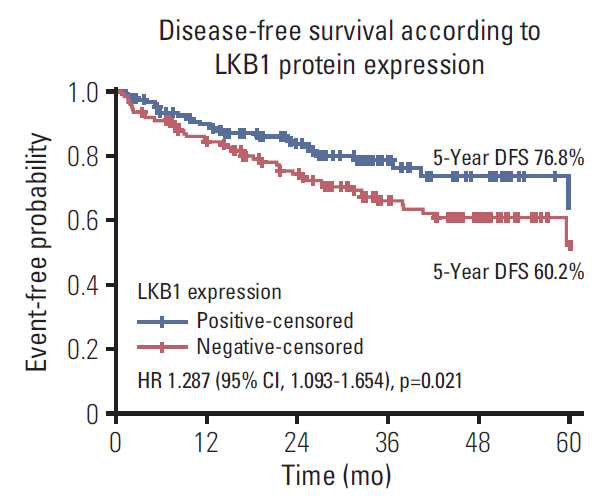
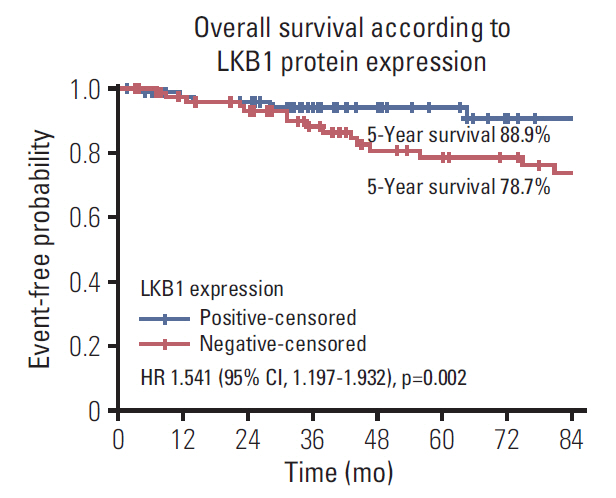
LKB1 expression loss was observed in 117 patients (44.7%) patients and correlated with right-sided located primaries (p=0.032), and pericolic lymph nodes involvement (p=0.003), BRAF(V600E) mutations (p=0.024), and TS mRNA expression (p=0.041). Patients with LKB1 expression loss experienced significantly lower disease-free survival (DFS) (hazard ratio [HR], 1.287; 95% confidence interval [CI], 1.093 to 1.654; p=0.021) and overall survival (OS) (HR, 1.541; 95% CI, 1.197 to 1.932; p=0.002), compared to patients with LKB1 expressing expressing tumors. Multivariate analysis revealed LKB1 expression loss as independent prognostic factor for both decreased DFS (HR, 1.217; 95% CI, 1.074 to 1.812; p=0.034) and decreased OS (HR, 1.467; 95% CI, 1.226 to 2.122; p=0.019).
CONCLUSION
Loss of tumoral LKB1 protein expression, constitutes an adverse prognostic factor in patients with operable CC.
MeSH Terms
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
Article2. Boland GM, Chang GJ, Haynes AB, Chiang YJ, Chagpar R, Xing Y, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013; 119:1593–601.
Article3. Schmoll HJ, Twelves C, Sun W, O'Connell MJ, Cartwright T, McKenna E, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on postrelapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 2014; 15:1481–92.
Article4. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372:2509–20.5. Shorning BY, Clarke AR. LKB1 loss of function studied in vivo. FEBS Lett. 2011; 585:958–66.
Article6. Forster LF, Defres S, Goudie DR, Baty DU, Carey FA. An investigation of the Peutz-Jeghers gene (LKB1) in sporadic breast and colon cancers. J Clin Pathol. 2000; 53:791–3.
Article7. Lee SW, Lin HK. A new mechanism for LKB1 activation. Mol Cell Oncol. 2018; 5:e1035691.
Article8. Lee SM, Choi JE, Na YK, Lee EJ, Lee WK, Choi YY, et al. Genetic and epigenetic alterations of the LKB1 gene and their associations with mutations in TP53 and EGFR pathway genes in Korean non-small cell lung cancers. Lung Cancer. 2013; 81:194–9.
Article9. Carretero J, Medina PP, Pio R, Montuenga LM, Sanchez-Cespedes M. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene. 2004; 23:4037–40.
Article10. Xiao J, Zou Y, Chen X, Gao Y, Xie M, Lu X, et al. The prognostic value of decreased LKB1 in solid tumors: a meta-analysis. PLoS One. 2016; 11:e0152674.
Article11. Souglakos J, Philips J, Wang R, Marwah S, Silver M, Tzardi M, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. 2009; 101:465–72.
Article12. Benlloch S, Paya A, Alenda C, Bessa X, Andreu M, Jover R, et al. Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real-time chemistry methodology. J Mol Diagn. 2006; 8:540–3.13. Saridaki Z, Tzardi M, Papadaki C, Sfakianaki M, Pega F, Kalikaki A, et al. Impact of KRAS, BRAF, PIK3CA mutations, PTEN, AREG, EREG expression and skin rash in ≥2 line cetuximab-based therapy of colorectal cancer patients. PLoS One. 2011; 6:e15980.14. Cushman-Vokoun AM, Stover DG, Zhao Z, Koehler EA, Berlin JD, Vnencak-Jones CL. Clinical utility of KRAS and BRAF mutations in a cohort of patients with colorectal neoplasms submitted for microsatellite instability testing. Clin Colorectal Cancer. 2013; 12:168–78.
Article15. Papadaki C, Mavroudis D, Trypaki M, Koutsopoulos A, Stathopoulos E, Hatzidaki D, et al. Tumoral expression of TXR1 and TSP1 predicts overall survival of patients with lung adenocarcinoma treated with first-line docetaxel-gemcitabine regimen. Clin Cancer Res. 2009; 15:3827–33.
Article16. Rowan A, Churchman M, Jefferey R, Hanby A, Poulsom R, Tomlinson I. In situ analysis of LKB1/STK11 mRNA expression in human normal tissues and tumours. J Pathol. 2000; 192:203–6.17. Gan RY, Li HB. Recent progress on liver kinase B1 (LKB1): expression, regulation, downstream signaling and cancer suppressive function. Int J Mol Sci. 2014; 15:16698–718.18. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009; 9:563–75.
Article19. Yang JY, Jiang SH, Liu DJ, Yang XM, Huo YM, Li J, et al. Decreased LKB1 predicts poor prognosis in pancreatic ductal adenocarcinoma. Sci Rep. 2015; 5:10575.
Article20. Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res. 2002; 8:2085–90.21. Sun J, Ling B, Xu X, Ma R, Li G, Cao X, et al. Decreased expression of tumor-suppressor gene LKB1 correlates with poor prognosis in human gastric cancer. Anticancer Res. 2016; 36:869–75.22. He TY, Tsai LH, Huang CC, Chou MC, Lee H. LKB1 loss at transcriptional level promotes tumor malignancy and poor patient outcomes in colorectal cancer. Ann Surg Oncol. 2014; 21 Suppl 4:S703–10.
Article23. Koivunen JP, Kim J, Lee J, Rogers AM, Park JO, Zhao X, et al. Mutations in the LKB1 tumour suppressor are frequently detected in tumours from Caucasian but not Asian lung cancer patients. Br J Cancer. 2008; 99:245–52.
Article24. Donada M, Bonin S, Nardon E, De Pellegrin A, Decorti G, Stanta G. Thymidilate synthase expression predicts longer survival in patients with stage II colon cancer treated with 5-flurouracil independently of microsatellite instability. J Cancer Res Clin Oncol. 2011; 137:201–10.
Article25. Liang J, Jiang T, Yao RY, Liu ZM, Lv HY, Qi WW. The combination of ERCC1 and XRCC1 gene polymorphisms better predicts clinical outcome to oxaliplatin-based chemotherapy in metastatic colorectal cancer. Cancer Chemother Pharmacol. 2010; 66:493–500.
Article26. Lee KS, Kwak Y, Nam KH, Kim DW, Kang SB, Choe G, et al. Favorable prognosis in colorectal cancer patients with coexpression of c-MYC and ss-catenin. BMC Cancer. 2016; 16:730.
Article27. Li P, Zhou H, Zhu X, Ma G, Liu C, Lin B, et al. High expression of NEDD9 predicts adverse outcomes of colorectal cancer patients. Int J Clin Exp Pathol. 2014; 7:2565–70.28. Calles A, Sholl LM, Rodig SJ, Pelton AK, Hornick JL, Butaney M, et al. Immunohistochemical loss of LKB1 is a biomarker for more aggressive biology in KRAS-mutant lung adenocarcinoma. Clin Cancer Res. 2015; 21:2851–60.
Article29. Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007; 26:7825–32.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognosis and Recurrence Pattern of Right- and Left-Sided Colon Cancer in Stage II, Stage III, and Liver Metastasis After Curative Resection
- The Clinical and Histopathologic Features according to Loss of LKB1 Protein Expression on Primary Lung Cancer
- Tumor Suppressor Serine/Threonine Kinase LKB1 Expression, Not Kinase Activity, Increased in the Vascular Smooth Muscle Cells and Neointima in the Rat Carotid Artery Injury Model
- Long-term Outcomes of Laparoscopic Surgery for Colorectal Cancer
- Survival and recurrence in stage II endometrial cancers in relation to uterine risk stratification after introduction of lymph node resection and omission of postoperative radiotherapy: a Danish Gynecological Cancer Group Study