Korean Circ J.
2010 Nov;40(11):552-557. 10.4070/kcj.2010.40.11.552.
Tumor Suppressor Serine/Threonine Kinase LKB1 Expression, Not Kinase Activity, Increased in the Vascular Smooth Muscle Cells and Neointima in the Rat Carotid Artery Injury Model
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea. jojeong@cnu.ac.kr
- 2Division of Cardiology, Department of Internal Medicine, Eulji University Hospital, Daejeon, Korea.
- 3Department of Pathology, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 1826173
- DOI: http://doi.org/10.4070/kcj.2010.40.11.552
Abstract
- BACKGROUND AND OBJECTIVES
Vascular smooth muscle cell (VSMC) proliferation is responsible for the restenosis of previously inserted coronary stents. Angiotensin II (Ang II) is known to regulate VSMC proliferation. LKB1, a serine/threonine kinase, interacts with the p53 pathway and acts as a tumor suppressor.
MATERIALS AND METHODS
We assessed the association of Ang II and the expression of LKB1 in primary cultured murine VSMCs and neointima of the Sprague Dawley rat carotid artery injury model. We created carotid balloon injuries and harvested the injured carotid arteries 14 days after the procedure.
RESULTS
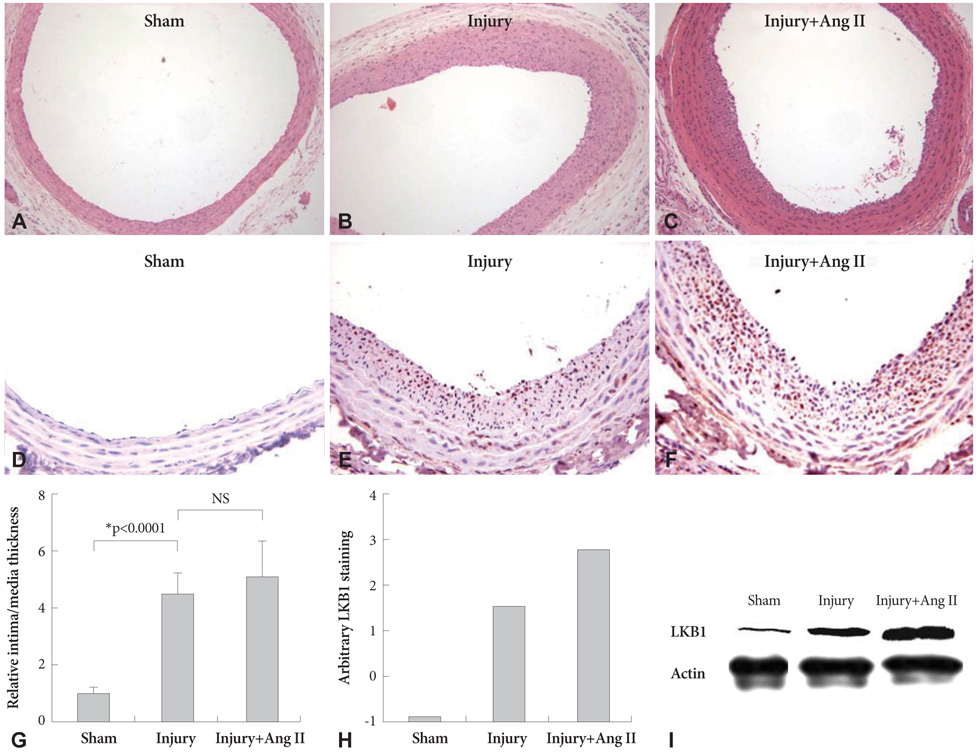
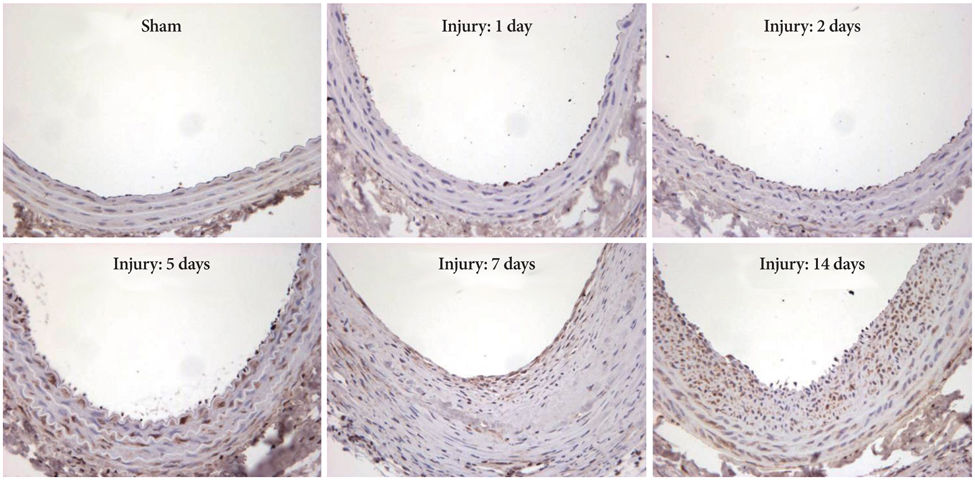
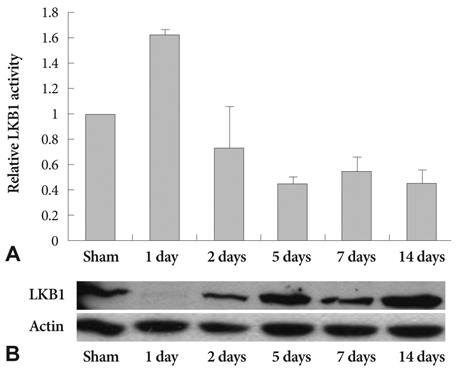
Ang II increased LKB1 expression in a time-dependent manner and peaked at an Ang II concentration of 10(-7) mole/L in VSMCs. In the animal experiment, neointima was markedly increased after balloon injury compared to the control group. Immunohistochemical studies showed that LKB1 expression increased according to neointima thickness. Ang II augmented LKB1 expression after the injury. Western blot analysis of LKB1 with carotid artery lysate revealed the same pattern as LKB1 immunohistochemistry. Increased LKB1 expression started at 5 days after the balloon injury, and peaked at 14 days after the injury. Although LKB1 expression was increased after the injury, LKB1 kinase activity was not increased. Ang II or balloon-injury increased the expression of LKB1 although the LKB1 activity was reduced.
CONCLUSION
Ang II increased LKB1 expression in VSMCs and neointima. These findings were not kinase dependant.
MeSH Terms
Figure
Reference
-
1. Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983. 49:208–215.2. Dussaillant GR, Mintz GS, Pichard AD, et al. Small stent size and intimal hyperplasia contribute to restenosis: a volumetric intravascular ultrasound analysis. J Am Coll Cardiol. 1995. 26:720–724.3. Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996. 94:1247–1254.4. Schwartz SM, deBlois D, O'Brien ER. The intima: soil for atherosclerosis and restenosis. Circ Res. 1995. 77:445–465.5. Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998. 391:184–187.6. Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998. 18:38–43.7. Ylikorkala A, Rossi DJ, Korsisaari N, et al. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science. 2001. 293:1323–1326.8. Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997. 88:323–331.9. Sherr CJ. Mammalian G1 cyclins and cell cycle progression. Proc Assoc Am Physicians. 1995. 107:181–186.10. Tiainen M, Vaahtomeri K, Ylikorkala A, Makela TP. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1). Hum Mol Genet. 2002. 11:1497–1504.11. Tiainen M, Ylikorkala A, Makela TP. Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest. Proc Natl Acad Sci U S A. 1999. 96:9248–9251.12. Karuman P, Gozani O, Odze RD, et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001. 7:1307–1319.13. Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996. 98:2277–2283.14. Kwon JS, Park NK, Jeong IH, et al. A slight variation in the age of rats commonly used as a carotid artery injury model results in a large difference in neointima formation. Korean Circ J. 2007. 37:78–83.15. Kwon JS, Park SS, Kim YG, et al. Perivascular delivery of paclitaxel with F-127 pluronic gel inhibits neointimal hyperplasia in a rat carotid injury model. Korean Circ J. 2005. 35:221–227.16. Joe JH, Lim KS, Jin JY, Kim KS. Effect of udenafil vascular smooth muscle cell proliferation and neointimal hyperplasia in rat carotid artery injury model. Korean Circ J. 2008. 38:320–324.17. Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury: I. smooth muscle growth in the absence of endothelium. Lab Invest. 1983. 49:327–333.18. Sharpless NE, Ramsey MR, Balasubramanian P, Castrillon DH, DePinho RA. The differential impact of p16(INK4a) or p19(ARF) deficiency on cell growth and tumorigenesis. Oncogene. 2004. 23:379–385.19. Kim DW, Chung HK, Park KC, et al. Tumor suppressor LKB1 inhibits activation of signal transducer and activator of transcription 3 (STAT3) by thyroid oncogenic tyrosine kinase rearranged in transformation (RET)/papillary thyroid carcinoma (PTC). Mol Endocrinol. 2007. 21:3039–3049.20. Nagata D, Takeda R, Sata M, et al. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004. 110:444–451.21. Kojima K, Motoshima H, Tsutsumi A, et al. Rottlerin activates AMPK possibly through LKB1 in vascular cells and tissues. Biochem Biophys Res Commun. 2008. 376:434–438.22. Chan AY, Dyck JR. Activation of AMP-activated protein kinase (AMPK) inhibits protein synthesis: a potential strategy to prevent the development of cardiac hypertrophy. Can J Physiol Pharmacol. 2005. 83:24–28.23. Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004. 279:32771–32779.24. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation: AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006. 574:63–71.25. Marignani PA, Kanai F, Carpenter CL. LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest. J Biol Chem. 2001. 276:32415–32418.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of a Novel Putative Protein Serine / Threonine Kinase, PK38, in Normal Human Keratinocytes
- Liver Kinase B1 Mediates Its Anti-Tumor Function by Binding to the N-Terminus of Malic Enzyme 3
- Effect of Udenafil on Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia in Rat Carotid Artery Injury Model
- LKB1/STK11 Tumor Suppressor Reduces Angiogenesis by Directly Interacting with VEGFR2 in Tumorigenesis
- Bortezomib Reduces Neointimal Hyperplasia in a Rat Carotid Artery Injury Model