Nat Prod Sci.
2019 Sep;25(3):200-207. 10.20307/nps.2019.25.3.200.
Albizzia julibrissin Suppresses Testosterone-induced Benign Prostatic Hyperplasia by Regulating 5α-Reductase Type 2 – Androgen Receptor Pathway
- Affiliations
-
- 1Department of Veterinary Medicine & Institute of Veterinary Science, Chungnam National University, Daejeon, Republic of Korea. jyjung@cnu.ac.kr
- 2Center for Vascular Research, Institute for Basic Science (IBS), Daejeon, Republic of Korea.
- 3Samsung Biomedical Research Institute, Seoul, Republic of Korea.
- 4Department of Pharmacy, Chungnam National University, Daejeon, Republic of Korea.
- KMID: 2459960
- DOI: http://doi.org/10.20307/nps.2019.25.3.200
Abstract
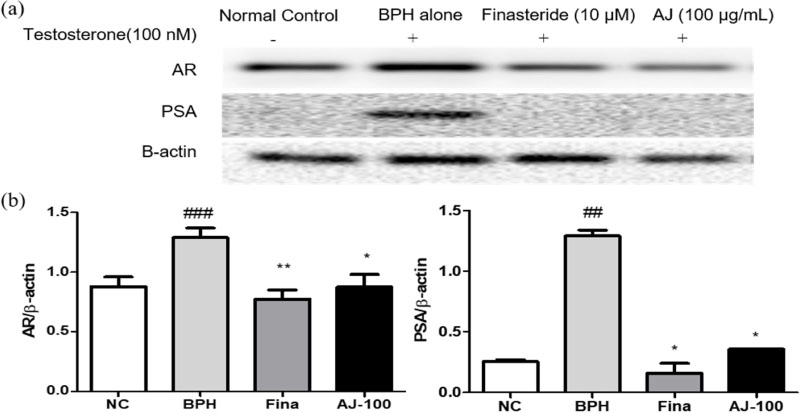
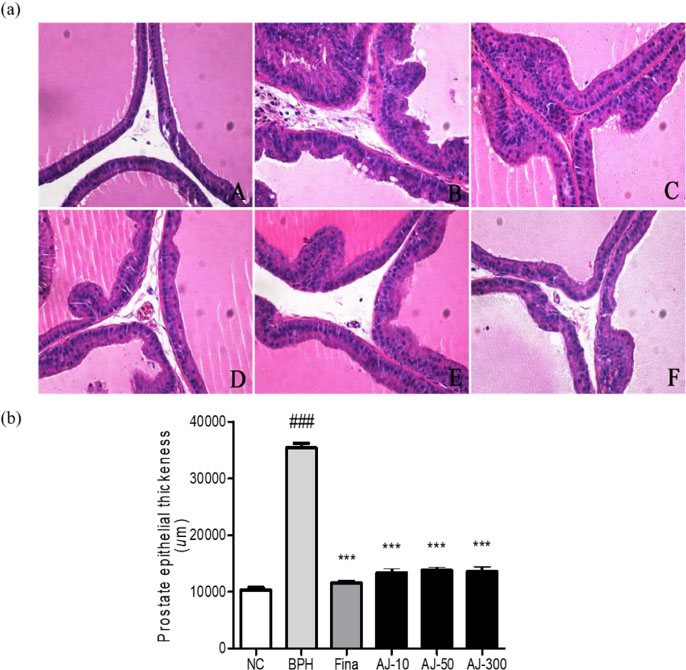
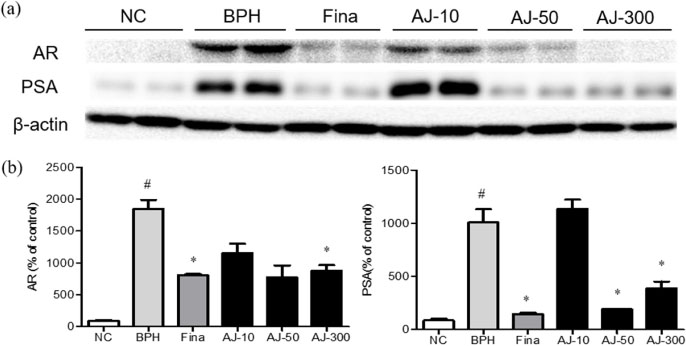
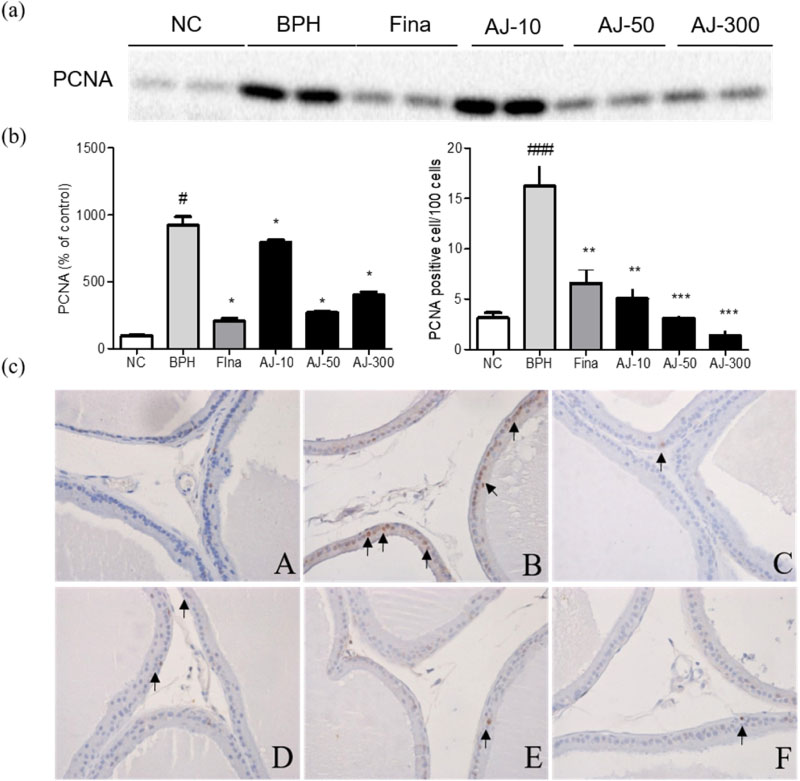
- Albizzia julibrissin (AJ) is an herbal medicine that shows low toxicity, promotes promoting blood circulation and mitigates the inflammation and has mild side effects. Benign prostate hyperplasia (BPH) is one of the most common diseases that occurs in older males and often results in lower urinary tract symptoms. This study was conducted to evaluate the protective effect of AJ against BPH using LNCaP cells and Sprague Dawley rats treated with testosterone. Treatment with AJ extract reduced the expression of androgen receptor (AR) and prostate-specific antigen (PSA) in vitro. In vivo, rats were divided into 6 groups: 1 (Normal Control); 2 (Testosterone propionate (TP) alone); 3 (TP + finasteride); 4 (TP + AJ 10 mg/kg); 5 (TP + AJ 50 mg/kg); 6 (TP + AJ 300 mg/kg). The groups treated with AJ showed reduced the relative prostate weights and BPH-related proteins were altered, with decreased AR, PSA and proliferating cell nuclear antigen (PCNA) observed by western blot. Histopathological analysis revealed the therapeutic effect of AJ, with a decreased thickness of epithelial cells and reduced level of PCNA and 5α-reductase type 2. These results suggest that AJ extract could ameliorate testosterone-induced benign prostatic hyperplasia.
Keyword
MeSH Terms
-
Albizzia*
Animals
Blood Circulation
Blotting, Western
Diethylpropion
Epithelial Cells
Herbal Medicine
Humans
Hyperplasia
In Vitro Techniques
Inflammation
Lower Urinary Tract Symptoms
Male
Proliferating Cell Nuclear Antigen
Prostate
Prostate-Specific Antigen
Prostatic Hyperplasia*
Rats
Rats, Sprague-Dawley
Receptors, Androgen*
Testosterone
Weights and Measures
Diethylpropion
Proliferating Cell Nuclear Antigen
Prostate-Specific Antigen
Receptors, Androgen
Testosterone
Figure
Reference
-
1. Higichi H, Kinjo J, Nohara T. Chem Pharm Bull (Tokyo). 1992; 40:829–831.2. Lv JS, Zhang LN, Song YZ, Wang XF, Chu XZ. Int Biodeterior Biodegrad. 2011; 65:258–264.3. Lau CS, Carrier DJ, Beitle RR, Bransby DI, Howard LR, Lay JO Jr, Liyanage R, Clausen EC. Bioresour Technol. 2007; 98:429–435.4. Zheng L, Zheng J, Zhao Y, Wang B, Wu L, Liang H. Bioorg Med Chem Lett. 2006; 16:2765–2768.5. Kang TH, Jeong SJ, Kim NY, Higuchi R, Kim YC. J Ethnopharmacol. 2000; 71:321–323.6. Kim JH, Kim SY, Lee SY, Jang CG. Pharmacol Biochem Behav. 2007; 87:41–47.7. Gupta RS, Chaudhary R, Yadav RK, Verma SK, Dobhal MP. J Ethnopharmacol. 2005; 96:31–36.8. Kokila K, Priyadharshini SD, Sujatha V. Int J Pharm Pharm Sci. 2013; 5:70–73.9. Priest R, Garzotto M, Kaufman J. Tech Vasc Interv Radiol. 2012; 15:261–264.10. Sarma AB, Wei JT. N Engl J Med. 2012; 367:248–257.
Article11. Miller J, Tarter TH. Clin Interv Aging. 2009; 4:251–258.12. Nickel JC. Urol Clin North Am. 2008; 35:109–115.13. Kramer G, Marberger M. Curr Opin Urol. 2006; 16:25–29.
Article14. Choi HM, Jung Y, Park J, Kim HL, Youn DH, Kang JW, Jeong MY, Lee JH, Yang WM, Lee SG, Ahn KS, Um JY. Sci Rep. 2016; 6:31906.15. Azzouni F, Godoy A, Li Y, Mohler J. Adv Urol. 2012; 2012:530121.16. Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, Russell D, Tindall D. J Urol. 2004; 172:1399–1403.17. Goldenberg L, So A, Fleshner N, Rendon R, Drachenberg D, Elhilali M. Can Urol Assoc J. 2009; 3:S109–S114.18. Lonergan PE, Tindall DJ. J Carcinog. 2011; 10:20.19. Sreenivasulu K, Nandeesha H, Dorairajn LN, Rajesh NG. Indian J Pathol Microbiol. 2019; 62:99–102.20. Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Am J Pathol. 2013; 182:1942–1949.21. Bae JS, Park HS, Park JW, Li SH, Chun YS. J Nat Med. 2012; 66:476–485.22. Lepor H. Rev Urol. 2004; 6 Suppl 9:S3–S10.23. Velonas VM, Woo HH, dos Remedios CG, Assinder SJ. Int J Mol Sci. 2013; 14:11034–11060.24. Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somervile MC, Rittmaster RS. Eur Urol. 2008; 54:1379–1384.25. Schmidt LJ, Tindall DJ. J Steroid Biochem Mol Biol. 2011; 125:32–38.26. Zhong WD, Peng J, He HC, Wu D, Han ZD, Bi XC, Dai QS. Clin Invest Med. 2008; 31:E8–E15.27. Kapoor A. Can J Urol. 2012; 19:10–17.28. Djavan B, Marberger M. T. Eur Urol. 1999; 36:1–13.29. Liu WK, Xu SX, Che CT. Life Sci. 2000; 67:1297–1306.30. Hussein SA, Hashim AN, Barakat HH, Jose J, Lindequist U, Nawwar MA. Pharmazie. 2006; 61:1034–1037.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 5alpha-Reductase
- Corni Fructus attenuates testosterone-induced benign prostatic hyperplasia by suppressing 5α-reductase and androgen receptor expression in rats
- Effect of Progesterone on COX-2 Expression and Proliferation of Prostate Stromal Cell
- Mixture of Corni Fructus and Schisandrae Fructus improves testosterone-induced benign prostatic hyperplasia through regulating 5α-reductase 2 and androgen receptor
- The Antihyperplastic Effect of Oral Catechin Ingestion in a Rat Model of Benign Prostatic Hyperplasia