J Breast Cancer.
2019 Sep;22(3):425-438. 10.4048/jbc.2019.22.e41.
The Role of Neutrophil-lymphocyte Ratio and Platelet-lymphocyte Ratio in Predicting Neoadjuvant Chemotherapy Response in Breast Cancer
- Affiliations
-
- 1Department of Surgery, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea. ab-lee@hanmail.net
- 2Department of Pathology, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2458863
- DOI: http://doi.org/10.4048/jbc.2019.22.e41
Abstract
- PURPOSE
The role of the host immunologic environment is crucial in cancer progression. Recent studies revealed that neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), are possible surrogate markers of outcome in various cancers. In breast cancer, the therapeutic effect of neoadjuvant chemotherapy (NAC) differs in patients, and higher response rate reflects better outcomes. This study aimed to determine the predictive value of peripheral blood NLR and PLR for NAC response along with their prognostic role in breast cancer. METHOD: A total of 105 patients with breast cancer treated with NAC between 2009 and 2017 were analyzed retrospectively. NAC response and prognosis (disease-free-survival [DFS], progression-free-survival [PFS] and overall survival [OS]) according to NLR and PLR were evaluated. According to the optimal cut-off values for NAC response obtained from receiver operating characteristic (ROC) curves, patients satisfying both low NLR and PLR levels (low-ratio group) were compared to those who did not (high-ratio group).
RESULTS
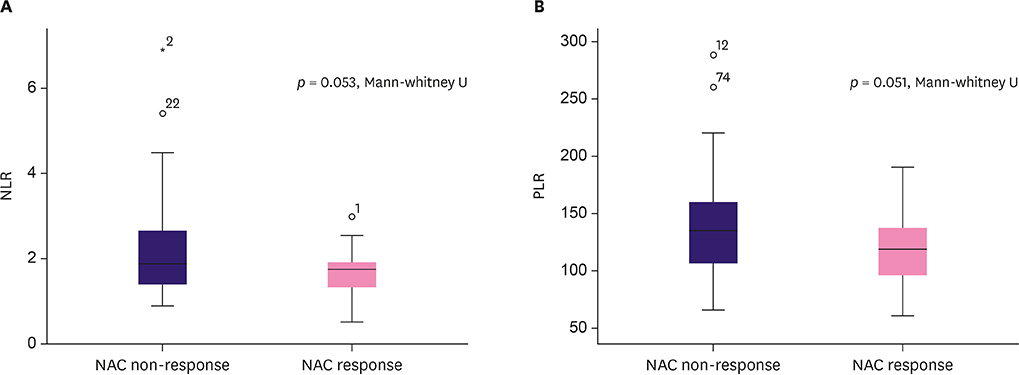
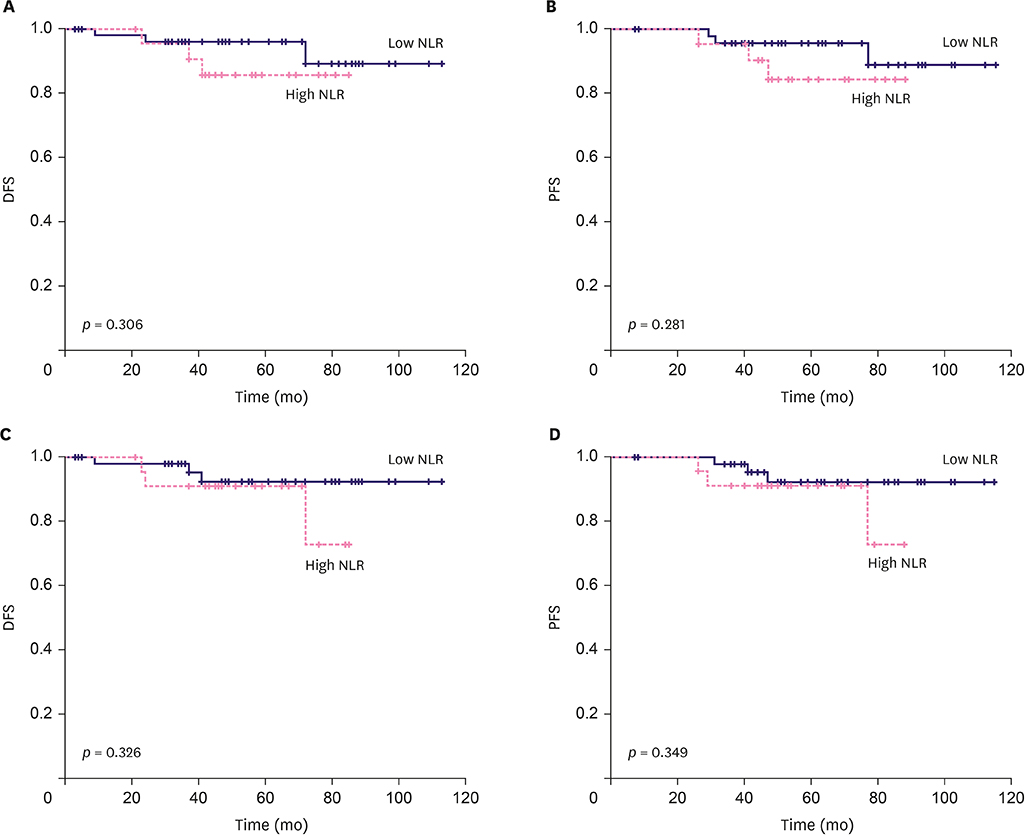
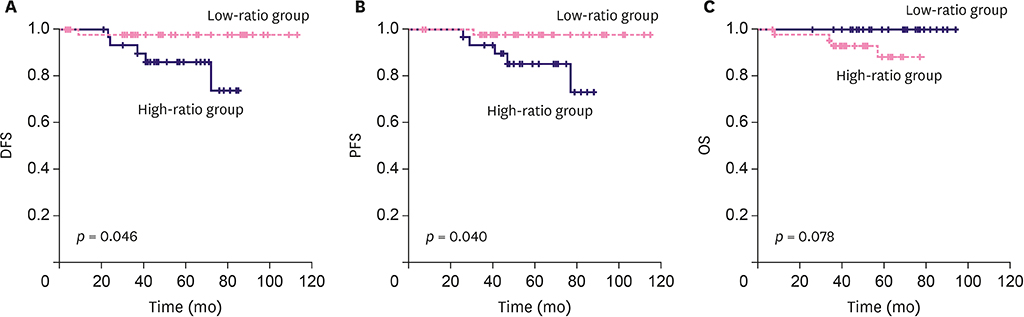
The NLR cut-off value was ≤ 2.21 (area under the ROC curve [AUC], 0.617; 95% confidence interval [CI], 0.517-0.710; p=0.030) with 94.1% sensitivity and 38.0% specificity. The PLR cut-off value was ≤ 143.36 (AUC, 0.618; 95% CI, 0.518-0.711; p = 0.040) with 85.3% sensitivity and 39.4% specificity. The low-ratio group demonstrated a better NAC response (p = 0.006) in multivariate analysis than the high-ratio group. The low-ratio group showed better DFS and PFS (p = 0.046 and p = 0.040, respectively) and longer OS (p = 0.078) in univariate survival analysis than the high-ratio group. In multivariate analysis, the low-ratio group had significantly better PFS (p = 0.049) and higher DFS (p = 0.054) than the high-ratio group.
CONCLUSIONS
The combination of NLR and PLR showed improved prediction of NAC response, revealing their potential as screening tools, significant prognostic role in breast cancer patients who receive NAC. Further studies with subgroup analysis, larger population and longer follow up duration are required.
Keyword
MeSH Terms
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Zer A, Rizel S, Braunstein R, Yerushalmi R, Hendler D, Neimann V, et al. Tailoring neoadjuvant chemotherapy for locally advanced breast cancer: a historical prospective study. Chemotherapy. 2012; 58:95–101.
Article3. Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer. 2011; 47:2084–2090.
Article4. Petit T, Wilt M, Velten M, Millon R, Rodier JF, Borel C, et al. Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Eur J Cancer. 2004; 40:205–211.
Article5. Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014; 148:467–476.
Article6. Stegner D, Dütting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res. 2014; 133:Suppl 2. S149–S157.
Article7. Paulsson J, Sjöblom T, Micke P, Pontén F, Landberg G, Heldin CH, et al. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am J Pathol. 2009; 175:334–341.
Article8. Seretis C, Seretis F, Lagoudianakis E, Politou M, Gemenetzis G, Salemis NS. Enhancing the accuracy of platelet to lymphocyte ratio after adjustment for large platelet count: a pilot study in breast cancer patients. Int J Surg Oncol. 2012; 2012:653608.
Article9. Krenn-Pilko S, Langsenlehner U, Thurner EM, Stojakovic T, Pichler M, Gerger A, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014; 110:2524–2530.
Article10. Tsung K, Meko JB, Peplinski GR, Tsung YL, Norton JA. IL-12 induces T helper 1-directed antitumor response. J Immunol. 1997; 158:3359–3365.11. Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017; 19:2.
Article12. Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T, et al. Platelet-lymphocyte ratio as a useful predictor of the therapeutic effect of neoadjuvant chemotherapy in breast cancer. PLoS One. 2016; 11:e0153459.
Article13. American Joint Committee on Cancer. Cancer Staging Manual Breast Cancer. 8th ed. Chicago: American Joint Committee on Cancer;2017.14. Park CK, Jung WH, Koo JS. Pathologic evaluation of breast cancer after neoadjuvant therapy. J Pathol Transl Med. 2016; 50:173–180.
Article15. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420:860–867.
Article16. Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013; 14:5237–5242.
Article17. Yao M, Liu Y, Jin H, Liu X, Lv K, Wei H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther. 2014; 7:1743–1752.18. Lee M, Kim SW, Nam EJ, Yim GW, Kim S, Kim YT. The impact of pretreatment thrombocytosis and persistent thrombocytosis after adjuvant chemotherapy in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2011; 122:238–241.
Article19. Heldin CH, Betsholtz C, Johnsson A, Nistér M, Ek B, Rönnstrand L, et al. Platelet-derived growth factor: mechanism of action and relation to oncogenes. J Cell Sci Suppl. 1985; 3:65–76.
Article20. Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002; 2:311–315.
Article21. Suzuki K, Aiura K, Ueda M, Kitajima M. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas. 2004; 29:132–140.
Article22. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013; 31:860–867.
Article23. Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015; 15:405–414.
Article24. Koh CH, Bhoo-Pathy N, Ng KL, Jabir RS, Tan GH, See MH, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015; 113:150–158.
Article25. Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013; 16:55–59.
Article26. Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013; 30:432.
Article27. Graziano V, Grassadonia A, Iezzi L, Vici P, Pizzuti L, Barba M, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. 2019; 44:33–38.
Article28. Kurebayashi J, Yamamoto Y, Tanaka K, Kohno N, Kurosumi M, Moriya T, et al. Significance of serum carcinoembryonic antigen and CA 15-3 in monitoring advanced breast cancer patients treated with systemic therapy: a large-scale retrospective study. Breast Cancer. 2003; 10:38–44.
Article29. Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol. 2016; 23:1104–1110.
Article30. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Neutrophil-to-Lymphocyte Ratio in Predicting Pathological Response for Resectable Non–Small Cell Lung Cancer Treated with Neoadjuvant Chemotherapy Combined with PD-1 Checkpoint Inhibitors
- Evaluation of the role of inflammatory blood markers in predicting the pathological response after neoadjuvant chemoradiation in patients with locally advanced rectal cancer
- Prognostic Significance of the Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Neuroendocrine Carcinoma
- Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer?
- Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Patients with Esophageal Squamous Cell Carcinoma Incorporating Hematological Biomarkers