Cancer Res Treat.
2022 Oct;54(4):1017-1029. 10.4143/crt.2021.1007.
The Role of Neutrophil-to-Lymphocyte Ratio in Predicting Pathological Response for Resectable Non–Small Cell Lung Cancer Treated with Neoadjuvant Chemotherapy Combined with PD-1 Checkpoint Inhibitors
- Affiliations
-
- 1Department of Lung Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin's Clinical Research Center for Cancer, Tianjin Lung Cancer Center, Tianjin, China
- KMID: 2534181
- DOI: http://doi.org/10.4143/crt.2021.1007
Abstract
- Purpose
The aim of our study was to investigate the value of baseline and preoperative neutrophil-to-lymphocyte ratio (NLR) in predicting the pathological response and disease-free survival (DFS) of neoadjuvant chemotherapy alone or combined with programmed cell death-1 (PD-1) checkpoint inhibitors in patients with resectable non‒small cell lung cancer (NSCLC).
Materials and Methods
Resectable NSCLC patients who underwent neoadjuvant chemotherapy alone or combined with PD-1 checkpoint inhibitors between January 2018 and January 2020 were included. Peripheral venous blood samples of the patients were collected within 3 days prior to the first neoadjuvant treatment and within 3 days prior to surgery.
Results
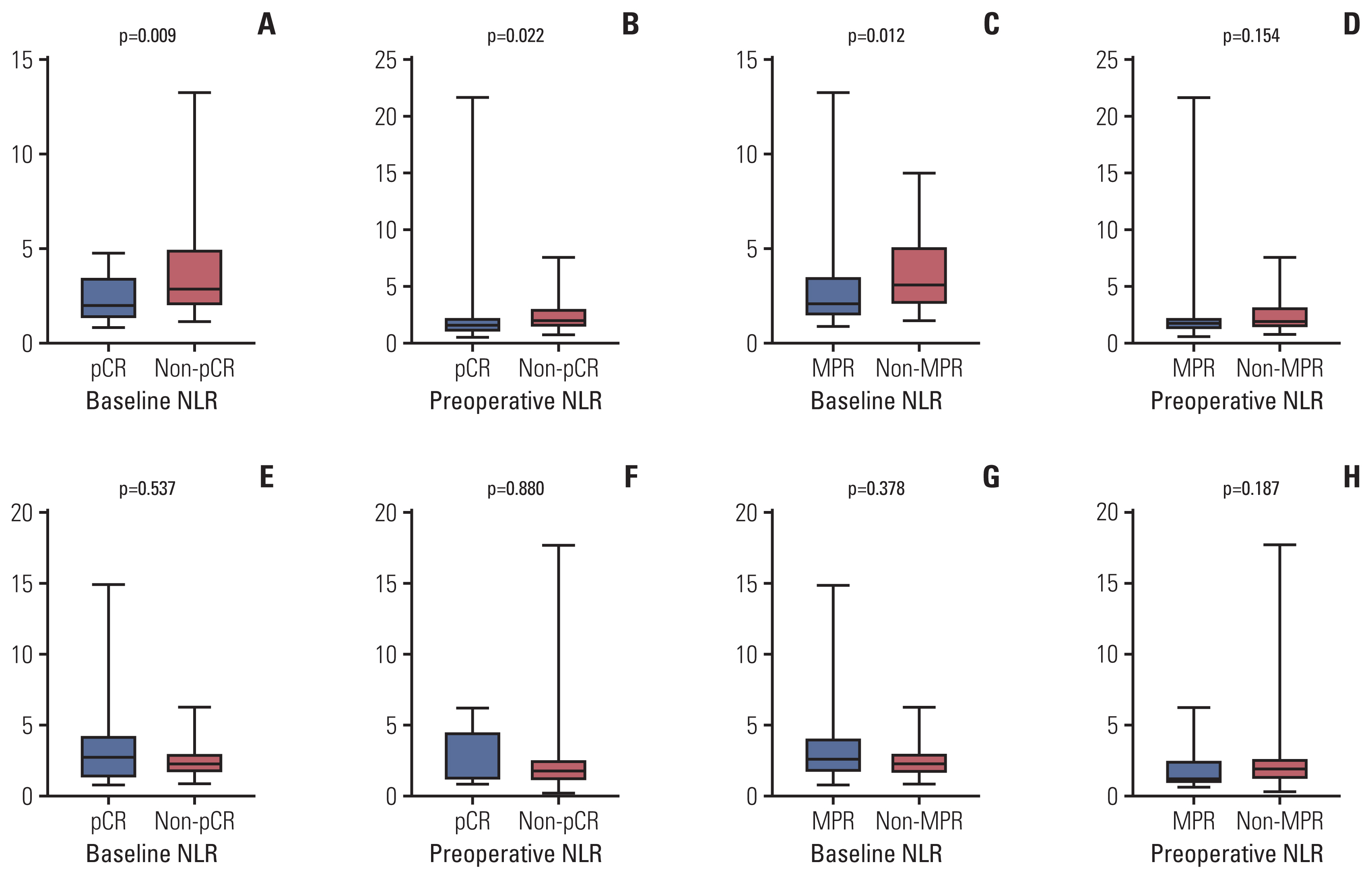
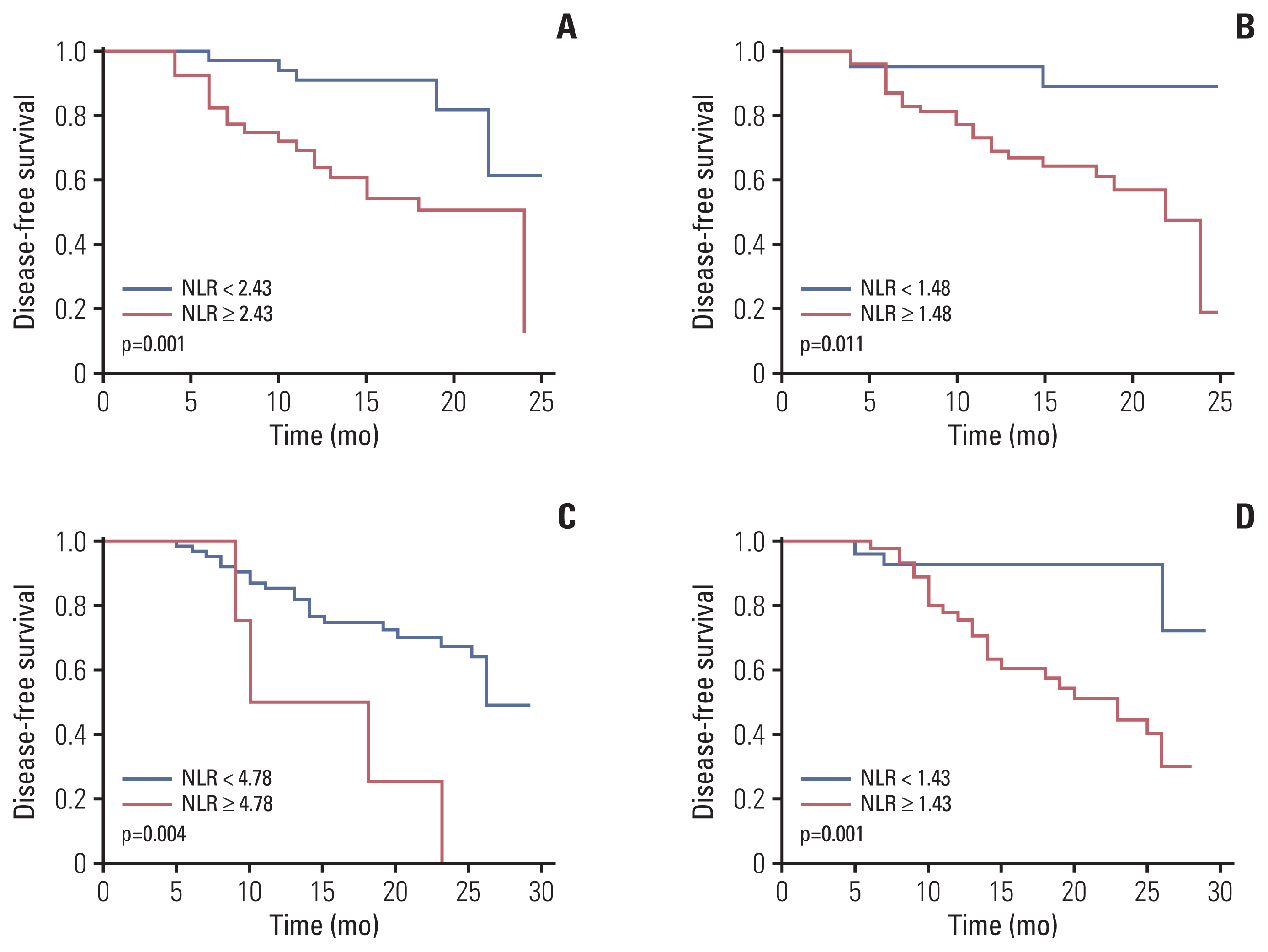
A total of 79 patients in neoadjuvant chemotherapy combined with PD-1 checkpoint inhibitors group and 89 patients in neoadjuvant chemotherapy alone group were included. Thirty-five point four percent of the patients achieved pathological complete response (pCR) in neoadjuvant chemotherapy combined with PD-1 checkpoint inhibitors group, whereas only 9.0% reached pCR in the group of neoadjuvant chemotherapy. High NLR level were correlated with poor pathological response and DFS in neoadjuvant chemotherapy or combined with PD-1 checkpoint inhibitors group. Multivariate analysis revealed that baseline NLR could independently predict pathological response and DFS in the neoadjuvant chemotherapy combined with PD-1 checkpoint inhibitors group.
Conclusion
High NLR level were correlated with poor pathological response and shorter DFS in patients with NSCLC undergoing neoadjuvant chemotherapy or combined with PD-1 checkpoint inhibitors. Meanwhile, baseline NLR could independently predict response to pathological response and DFS, revealing its potential as a screening tool in NSCLC patients who received neoadjuvant chemotherapy combined with PD-1 checkpoint inhibitors.
Keyword
Figure
Reference
-
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Liu L, Wei S. Research progress of KRAS mutation in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2018; 21:419–24.3. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017; 377:1919–29.
Article4. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015; 372:2018–28.
Article5. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell Lung Cancer. N Engl J Med. 2018; 378:2078–92.
Article6. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018; 379:2040–51.
Article7. Ling Y, Li N, Li L, Guo C, Wei J, Yuan P, et al. Different pathologic responses to neoadjuvant anti-PD-1 in primary squamous lung cancer and regional lymph nodes. NPJ Precis Oncol. 2020; 4:32.
Article8. Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016; 6:1382–99.
Article9. Yi JS, Ready N, Healy P, Dumbauld C, Osborne R, Berry M, et al. Immune activation in early-stage non-small cell lung cancer patients receiving neoadjuvant chemotherapy plus ipilimumab. Clin Cancer Res. 2017; 23:7474–82.
Article10. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100:57–70.
Article11. Russo A, Russano M, Franchina T, Migliorino MR, Aprile G, Mansueto G, et al. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with nivolu-mab in pretreated non-small cell lung cancer (NSCLC): a large retrospective multicenter study. Adv Ther. 2020; 37:1145–55.
Article12. Wang Y, Li Y, Chen P, Xu W, Wu Y, Che G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: a meta-analysis. Ann Transl Med. 2019; 7:433.
Article13. Pisters KM, Kris MG, Gralla RJ, Zaman MB, Heelan RT, Martini N. Pathologic complete response in advanced non-small-cell lung cancer following preoperative chemotherapy: implications for the design of future non-small-cell lung cancer combined modality trials. J Clin Oncol. 1993; 11:1757–62.
Article14. Betticher DC, Hsu Schmitz SF, Totsch M, Hansen E, Joss C, von Briel C, et al. Mediastinal lymph node clearance after docetaxelcisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003; 21:1752–9.
Article15. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–99.
Article16. Li Q, Zhou S, Liu S, Liu S, Yang H, Zhao L, et al. Treatment-related lymphopenia predicts pathologic complete response and recurrence in esophageal squamous cell carcinoma undergoing neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2019; 26:2882–9.
Article17. Heo J, Chun M, Noh OK, Oh YT, Suh KW, Park JE, et al. Sustaining blood lymphocyte count during preoperative chemoradiotherapy as a predictive marker for pathologic complete response in locally advanced rectal cancer. Cancer Res Treat. 2016; 48:232–9.
Article18. Fang P, Jiang W, Davuluri R, Xu C, Krishnan S, Mohan R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol. 2018; 128:584–90.
Article19. Cullinane C, Creavin B, O’Leary DP, O’Sullivan MJ, Kelly L, Redmond HP, et al. Can the neutrophil to lymphocyte ratio predict complete pathologic response to neoadjuvant breast cancer treatment? A systematic review and meta-analysis. Clin Breast Cancer. 2020; 20:e675–81.
Article20. Choi JE, Villarreal J, Lasala J, Gottumukkala V, Mehran RJ, Rice D, et al. Perioperative neutrophil:lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: a retrospective study. Cancer Med. 2015; 4:825–33.
Article21. Dal Bello MG, Filiberti RA, Alama A, Orengo AM, Mussap M, Coco S, et al. The role of CEA, CYFRA21–1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J Transl Med. 2019; 17:74.
Article22. Kojima H, Terada Y, Yasuura Y, Konno H, Mizuno T, Isaka M, et al. Prognostic impact of the number of involved lymph node stations in patients with completely resected non-small cell lung cancer: a proposal for future revisions of the N classification. Gen Thorac Cardiovasc Surg. 2020; 68:1298–304.
Article23. Wu Y, Chen J, Zhao L, Li Q, Zhu J, Yang H, et al. Prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma incorporating hematological biomarkers. Cancer Res Treat. 2021; 53:172–83.
Article24. Powell A, Chin C, Coxon AH, Chalishazar A, Christian A, Roberts SA, et al. Neutrophil to lymphocyte ratio as a predictor of response to neoadjuvant chemotherapy and survival in oesophageal adenocarcinoma. BJS Open. 2020; 4:416–23.
Article25. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016; 27:732–8.
Article26. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017; 106:1–7.
Article27. Zhang H, Xia H, Zhang L, Zhang B, Yue D, Wang C. Clinical significance of preoperative neutrophil-lymphocyte vs platelet-lymphocyte ratio in primary operable patients with non-small cell lung cancer. Am J Surg. 2015; 210:526–35.
Article28. Li A, He K, Guo D, Liu C, Wang D, Mu X, et al. Pretreatment blood biomarkers predict pathologic responses to neo-CRT in patients with locally advanced rectal cancer. Future Oncol. 2019; 15:3233–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exploring histological predictive biomarkers for immune checkpoint inhibitor therapy response in non–small cell lung cancer
- Perioperative immunotherapy in stage IB-III non-small cell lung cancer: a critical review of its rationale and considerations
- Immunotherapy for Non-small-cell Lung Cancer: Current Status and Future Obstacles
- Gastrointestinal cancer treatment with immune checkpoint inhibitors
- Current status of immune checkpoint inhibitors in treatment of non-small cell lung cancer