Cancer Res Treat.
2021 Jan;53(1):172-183. 10.4143/crt.2020.594.
Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Patients with Esophageal Squamous Cell Carcinoma Incorporating Hematological Biomarkers
- Affiliations
-
- 1State Key Laboratory of Oncology in South China, Collaborative Innovation Centre for Cancer Medicine, Guangdong Esophageal Cancer Institute, Guangzhou, China
- 2Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Department of Liver Surgery, Sun Yat-sen University Cancer Center, Guangzhou, China
- 4Department of Thoracic Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
- KMID: 2510659
- DOI: http://doi.org/10.4143/crt.2020.594
Abstract
- Purpose
This study aimed to develop a nomogram for predicting pathologic complete response (pCR) after neoadjuvant chemoradiotherapy (CRT) in patients with esophageal squamous cell carcinoma (ESCC) by integrating hematological biomarkers and clinicopathological characteristics.
Materials and Methods
Between 2003 and 2017, 306 ESCC patients who underwent neoadjuvant CRT followed by esophagectomy were analyzed. Besides clinicopathological factors, hematological parameters before, during, and after CRT were collected. Univariate and multivariate logistic regression analyses were performed to identify predictive factors for pCR. A nomogram model was built and internally validated.
Results
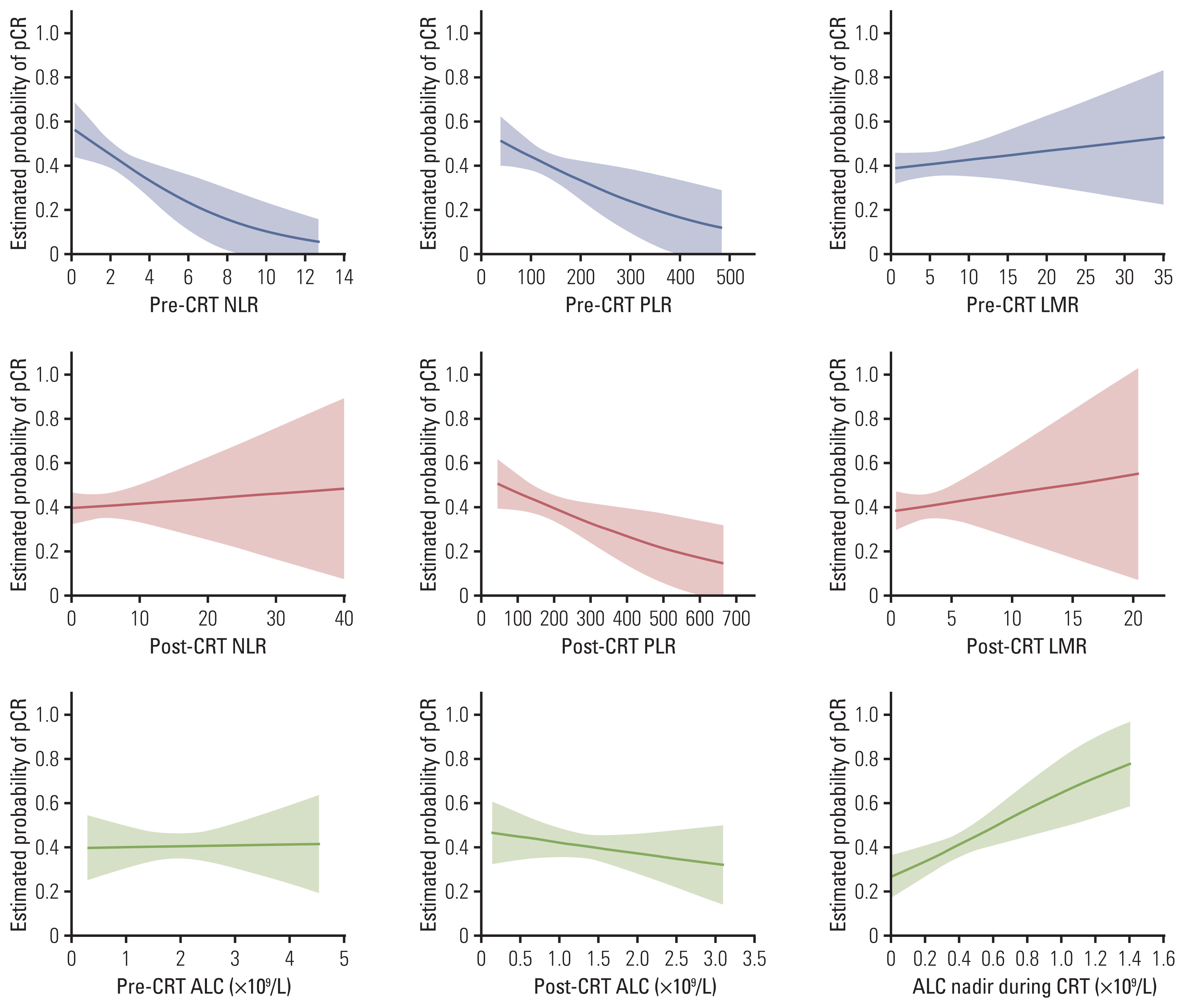
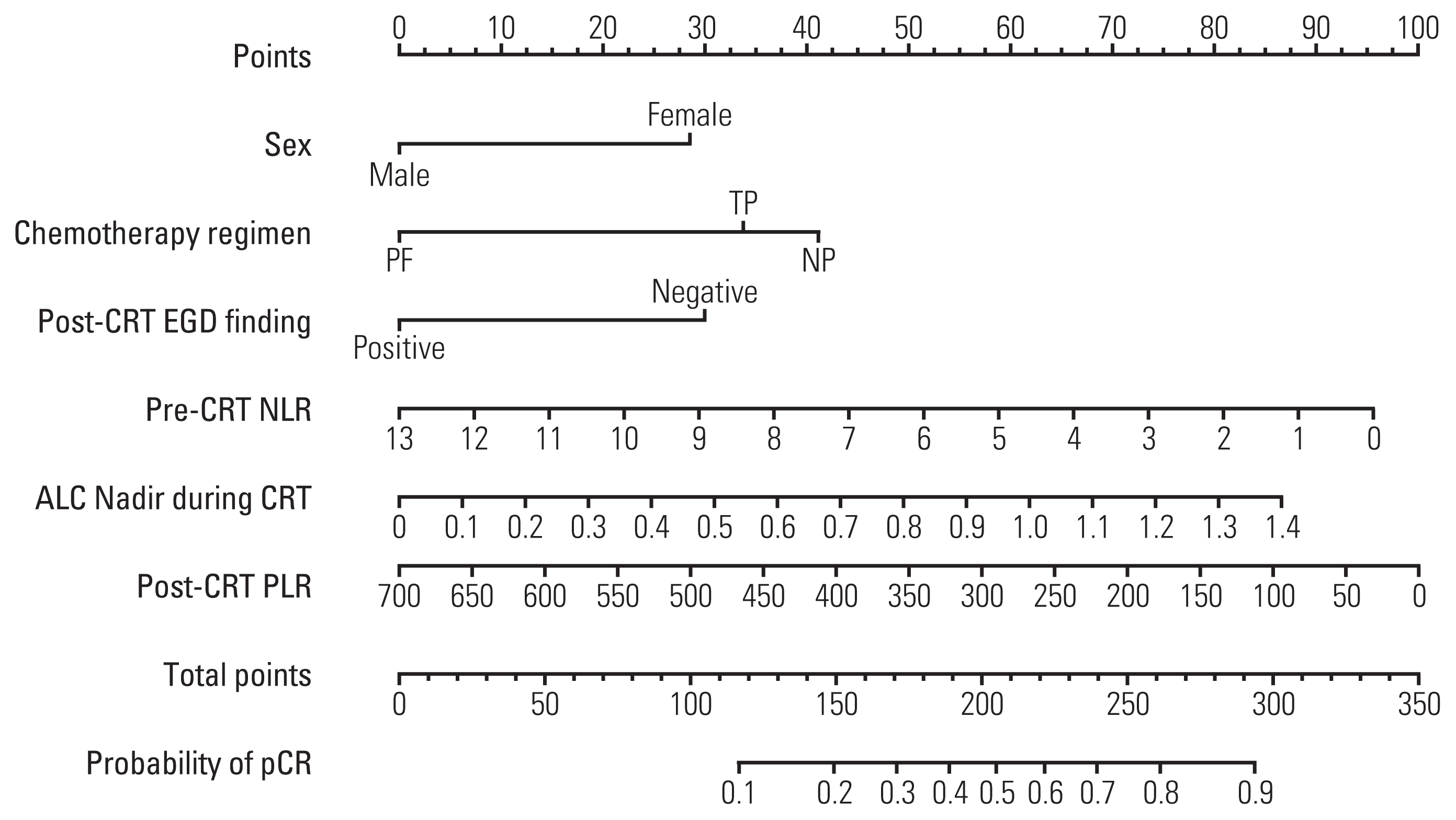
Absolute lymphocyte count (ALC), lymphocyte to monocyte ratio, albumin, hemoglobin, white blood cell, neutrophil, and platelet count generally declined, whereas neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) increased significantly following neoadjuvant CRT. After surgery, 124 patients (40.5%) achieved a pCR. The pCR group demonstrated significantly more favorable survival than the non-pCR group. On multivariate analysis, significant factors associated with pCR included sex, chemotherapy regimen, post-CRT endoscopic finding, pre-CRT NLR, ALC nadir during CRT, and post-CRT PLR, which were incorporated into the prediction model. The nomogram indicated good accuracy in predicting pCR, with a C-index of 0.75 (95% confidence interval, 0.71 to 0.78).
Conclusion
Female, chemotherapy regimen of cisplatin/vinorelbine, negative post-CRT endoscopic finding, pre-CRT NLR (≤ 2.1), ALC nadir during CRT (> 0.35 ×109/L), and post-CRT PLR (≤ 83.0) were significantly associated with pCR in ESCC patients treated with neoadjuvant CRT. A nomogram incorporating hematological biomarkers to predict pCR was developed and internally validated, showing good predictive performance.
Keyword
Figure
Cited by 1 articles
-
The Role of Neutrophil-to-Lymphocyte Ratio in Predicting Pathological Response for Resectable Non–Small Cell Lung Cancer Treated with Neoadjuvant Chemotherapy Combined with PD-1 Checkpoint Inhibitors
Xiaoyan Sun, Yingnan Feng, Bin Zhang, Wuhao Huang, Xiaoliang Zhao, Hua Zhang, Dongsheng Yue, Changli Wang
Cancer Res Treat. 2022;54(4):1017-1029. doi: 10.4143/crt.2021.1007.
Reference
-
References
1. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011; 12:681–92.
Article2. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012; 366:2074–84.
Article3. Shapiro J, van Lanschot JJ, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015; 16:1090–8.
Article4. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018; 36:2796–803.5. Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014; 32:2416–22.
Article6. Scheer RV, Fakiris AJ, Johnstone PA. Quantifying the benefit of a pathologic complete response after neoadjuvant chemoradiotherapy in the treatment of esophageal cancer. Int J Radiat Oncol Biol Phys. 2011; 80:996–1001.
Article7. van der Wilk BJ, Eyck BM, Spaander MC, Valkema R, Lagarde SM, Wijnhoven BP, et al. Towards an organ-sparing approach for locally advanced esophageal cancer. Dig Surg. 2019; 36:462–9.
Article8. Xi M, Yang Y, Zhang L, Yang H, Merrell KW, Hallemeier CL, et al. Multi-institutional analysis of recurrence and survival after neoadjuvant chemoradiotherapy of esophageal cancer: impact of histology on recurrence patterns and outcomes. Ann Surg. 2019; 269:663–70.9. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–99.
Article10. Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017; 99:128–35.
Article11. Shiraishi Y, Fang P, Xu C, Song J, Krishnan S, Koay EJ, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018; 128:154–60.
Article12. Zhou XL, Zhu WG, Zhu ZJ, Wang WW, Deng X, Tao WJ, et al. Lymphopenia in esophageal squamous cell carcinoma: relationship to malnutrition, various disease parameters, and response to concurrent chemoradiotherapy. Oncologist. 2019; 24:e677–86.
Article13. Fang P, Jiang W, Davuluri R, Xu C, Krishnan S, Mohan R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol. 2018; 128:584–90.
Article14. Li Q, Zhou S, Liu S, Liu S, Yang H, Zhao L, et al. Treatment-related lymphopenia predicts pathologic complete response and recurrence in esophageal squamous cell carcinoma undergoing neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2019; 26:2882–9.
Article15. Suzuki R, Lin SH, Wei X, Allen PK, Welsh JW, Byers LA, et al. Prognostic significance of pretreatment total lymphocyte count and neutrophil-to-lymphocyte ratio in extensive-stage small-cell lung cancer. Radiother Oncol. 2018; 126:499–505.
Article16. Xie D, Marks R, Zhang M, Jiang G, Jatoi A, Garces YI, et al. Nomograms predict overall survival for patients with small-cell lung cancer incorporating pretreatment peripheral blood markers. J Thorac Oncol. 2015; 10:1213–20.
Article17. Ishibashi Y, Tsujimoto H, Hiraki S, Kumano I, Yaguchi Y, Horiguchi H, et al. Prognostic value of preoperative systemic immunoinflammatory measures in patients with esophageal cancer. Ann Surg Oncol. 2018; 25:3288–99.
Article18. Hirahara N, Matsubara T, Kawahara D, Nakada S, Ishibashi S, Tajima Y. Prognostic significance of preoperative inflammatory response biomarkers in patients undergoing curative thoracoscopic esophagectomy for esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017; 43:493–501.
Article19. Heo J, Chun M, Noh OK, Oh YT, Suh KW, Park JE, et al. Sustaining blood lymphocyte count during preoperative chemoradiotherapy as a predictive marker for pathologic complete response in locally advanced rectal cancer. Cancer Res Treat. 2016; 48:232–9.
Article20. Deng W, Xu C, Liu A, van Rossum PS, Deng W, Liao Z, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol. 2019; 133:9–15.
Article21. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–44.
Article22. McLaren PJ, Bronson NW, Hart KD, Vaccaro GM, Gatter KM, Thomas CR Jr, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios can predict treatment response to neoadjuvant therapy in esophageal cancer. J Gastrointest Surg. 2017; 21:607–13.
Article23. Tustumi F, Takeda FR, Viyuela MS, da Cruz JB Junior, Brandao A, Sallum RA, et al. The value of cellular components of blood in the setting of trimodal therapy for esophageal cancer. J Surg Oncol. 2020; 121:784–94.
Article24. Conroy T, Etienne PL, Adenis A, Wagener DJ, Paillot B, Francois E, et al. Phase II trial of vinorelbine in metastatic squamous cell esophageal carcinoma. European Organization for Research and Treatment of Cancer Gastrointestinal Treat Cancer Cooperative Group. J Clin Oncol. 1996; 14:164–70.
Article25. Liu SL, Yang H, Zhang P, Zhang L, Zhao L, Luo LL, et al. Neoadjuvant chemoradiotherapy with cisplatin plus vinorelbine versus cisplatin plus fluorouracil for esophageal squamous cell carcinoma: a matched case-control study. Radiother Oncol. 2015; 116:262–8.
Article26. Ajani JA, Correa AM, Hofstetter WL, Rice DC, Blum MA, Suzuki A, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012; 23:2638–42.
Article27. Toxopeus EL, Nieboer D, Shapiro J, Biermann K, van der Gaast A, van Rij CM, et al. Nomogram for predicting pathologically complete response after neoadjuvant chemoradiotherapy for oesophageal cancer. Radiother Oncol. 2015; 115:392–8.
Article28. Chao YK, Chang HK, Tseng CK, Liu YH, Wen YW. Development of a nomogram for the prediction of pathological complete response after neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2017; 30:1–8.
Article29. Noordman BJ, Spaander MC, Valkema R, Wijnhoven BP, van Berge Henegouwen MI, Shapiro J, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018; 19:965–74.30. Borggreve AS, Goense L, van Rossum PS, Heethuis SE, van Hillegersberg R, Lagendijk JJ, et al. Preoperative prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal cancer using (18)F-FDG PET/CT and DW-MRI: a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2020; 106:998–1009.31. Beukinga RJ, Hulshoff JB, Mul VE, Noordzij W, Kats-Ugurlu G, Slart R, et al. Prediction of response to neoadjuvant chemotherapy and radiation therapy with baseline and restaging (18)F-FDG PET imaging biomarkers in patients with esophageal cancer. Radiology. 2018; 287:983–92.32. Azad TD, Chaudhuri AA, Fang P, Qiao Y, Esfahani MS, Chabon JJ, et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology. 2020; 158:494–505.
Article33. Zhou S, Zhao L, Liang Z, Liu S, Li Y, Liu S, et al. Indoleamine 2,3-dioxygenase 1 and programmed cell death-ligand 1 co-expression predicts poor pathologic response and recurrence in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. Cancers (Basel). 2019; 11:169.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Current Evidence on Neoadjuvant Therapy for Locally Advanced Esophageal Squamous Cell Carcinoma
- Adjuvant Therapy for Esophageal Squamous Cell Carcinoma
- Chemoradiotherapy for Esophageal Cancer
- Cutaneous Metastasis of Esophageal Squamous Cell Carcinoma Mimicking Benign Soft Tissue Tumor
- Response Evaluation after Neoadjuvant Chemoradiation by Positron Emission Tomography-Computed Tomography for Esophageal Squamous Cell Carcinoma