Investig Clin Urol.
2019 Sep;60(5):396-404. 10.4111/icu.2019.60.5.396.
Suppression of CD81 promotes bladder cancer cell invasion through increased matrix metalloproteinase expression via extracellular signal-regulated kinase phosphorylation
- Affiliations
-
- 1Department of Urology, Eulji University Hospital, Eulji University School of Medicine, Daejeon, Korea. jspark.uro@gmail.com
- 2Department of Microbiology and Immunology, Eulji University School of Medicine, Daejeon, Korea.
- KMID: 2455968
- DOI: http://doi.org/10.4111/icu.2019.60.5.396
Abstract
- PURPOSE
CD81 is a prognostic biomarker for high-grade bladder cancer (BC). In this study, we aimed to determine the functional mechanisms underlying the role of CD81 in BC progression.
MATERIALS AND METHODS
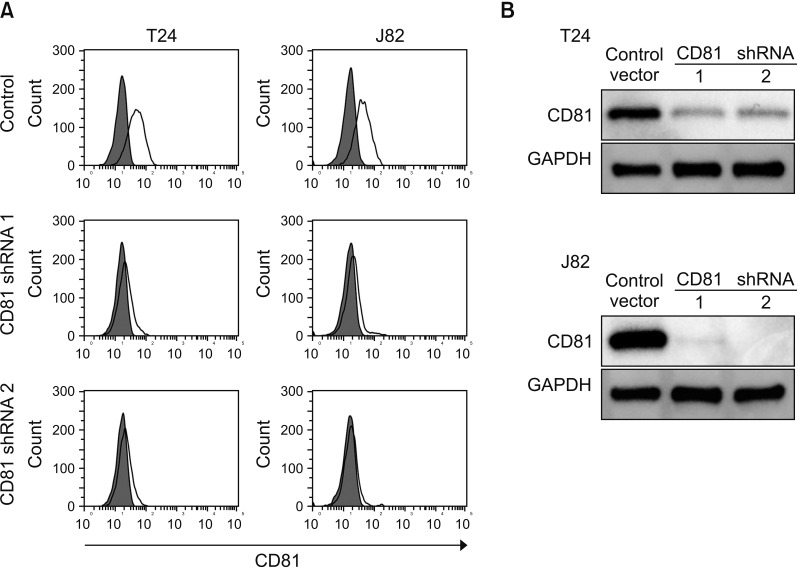
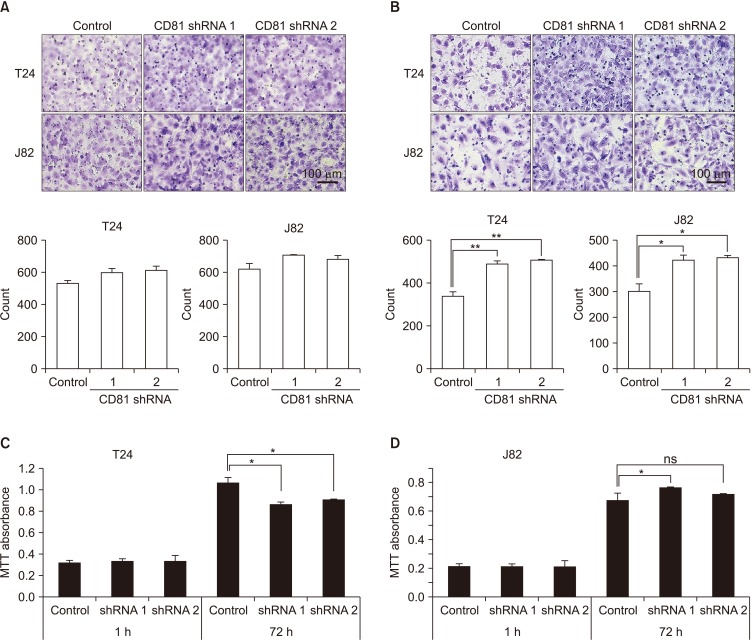
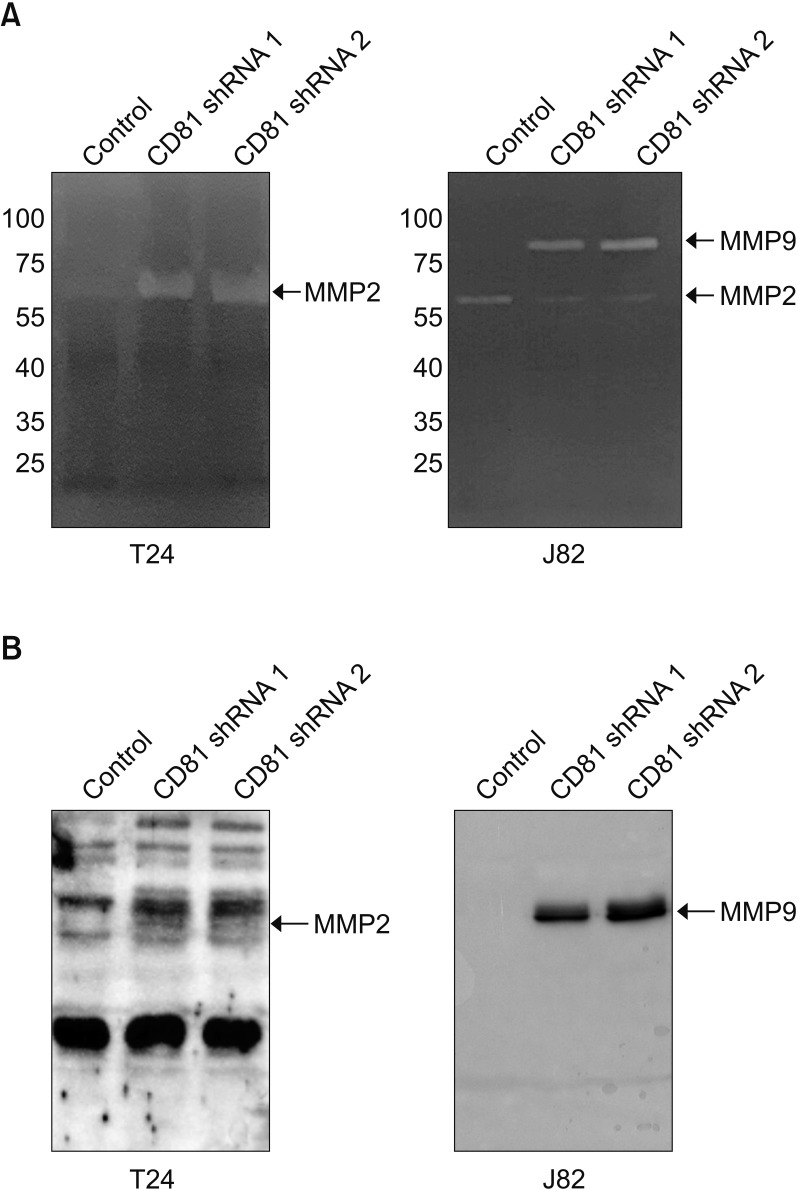
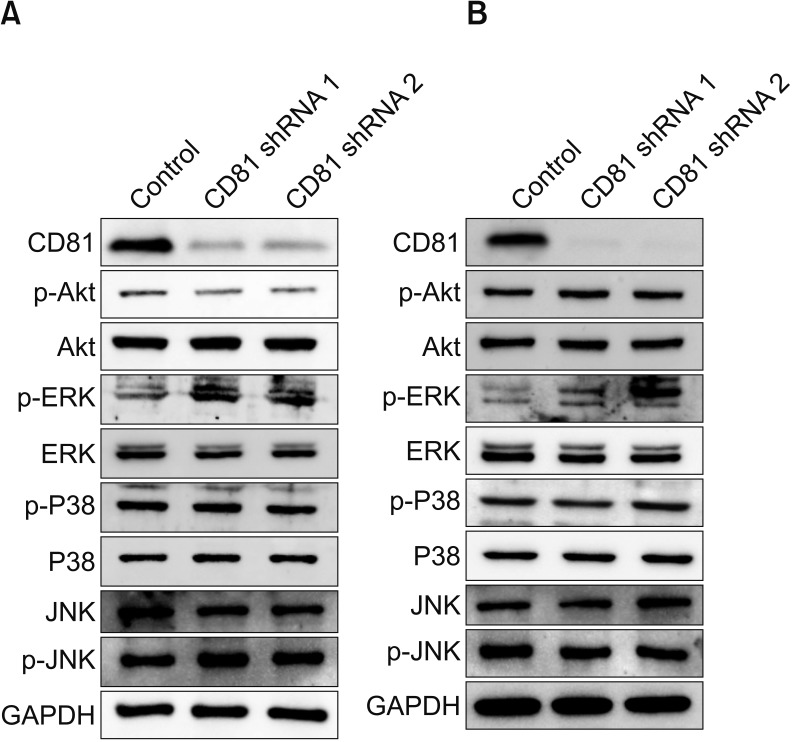
In two invasive BC cell lines (T24, J82), CD81 expression was suppressed by the transfection of lentiviral vectors including CD81-specific shRNAs, and then the migration and invasion of BC cells was analyzed. Enzymatic activity of matrix metalloproteinases (MMPs) was also analyzed by collagen-zymography. The expression of MMPs was confirmed by western blotting using culture supernatants from each cell line. Signaling pathways related to MMPs were investigated using various antibodies.
RESULTS
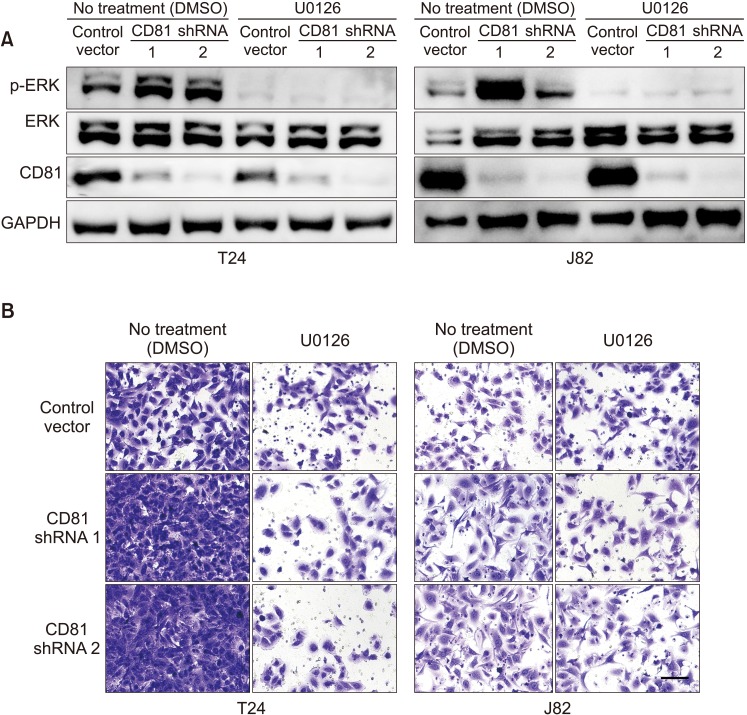
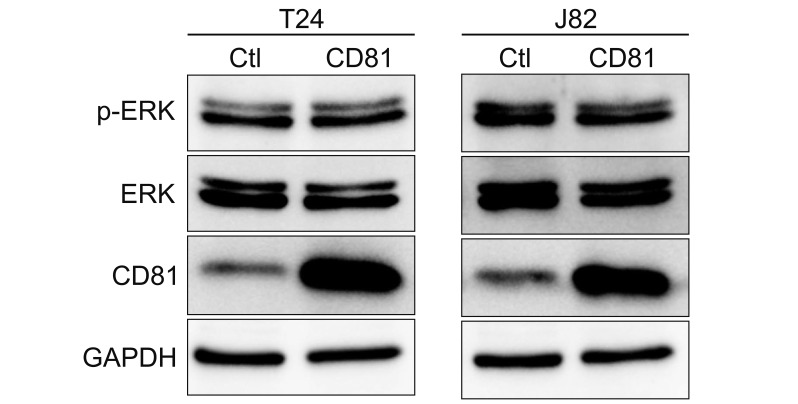
CD81 was successfully knocked down by shRNAs in T24 and J82 cell lines. While the migration of BC cells was not affected after the knockdown of CD81, the invasive activity was significantly increased in both cell lines. Zymography produced distinct bands using supernatants from CD81-knockdown cells, whereas only faint bands were observed with empty vector-transfected cells. We also observed an increased expression of MMPs, specifically MMP2 and 9, in the conditioned media from CD81-knockdown cells by western blotting. Mechanistically, the phosphorylation of extracellular signal-regulated kinase (ERK) was associated with the invasive activity of BC cells, while U0126 (an ERK inhibitor) reduced the invasive activity of CD81-knockdown BC cells.
CONCLUSIONS
Taken together, CD81 suppression promotes the invasive property of BC cells through MMP signaling via ERK phosphorylation. Our results suggest that the regulation of CD81 expression may have some therapeutic potential in BC.
Keyword
MeSH Terms
-
Antibodies
Antigens, CD81
Blotting, Western
Cell Line
Culture Media, Conditioned
Disease Progression
Extracellular Signal-Regulated MAP Kinases
Matrix Metalloproteinases
Phosphorylation*
Phosphotransferases*
RNA, Small Interfering
Transfection
Urinary Bladder Neoplasms*
Urinary Bladder*
Antibodies
Antigens, CD81
Culture Media, Conditioned
Extracellular Signal-Regulated MAP Kinases
Matrix Metalloproteinases
Phosphotransferases
RNA, Small Interfering
Figure
Reference
-
1. Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017; 3:524–548. PMID: 27918777.2. van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009; 56:430–442. PMID: 19576682.
Article3. Miyake M, Fujimoto K, Hirao Y. Active surveillance for non-muscle invasive bladder cancer. Investig Clin Urol. 2016; 57 Suppl 1:S4–S13.
Article4. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007; 178:2314–2330. PMID: 17993339.
Article5. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J. European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008; 54:303–314. PMID: 18468779.
Article6. Lee MS, Kim JH, Lee JS, Yun SJ, Kim WJ, Ahn H, et al. Prognostic significance of CREB-Binding protein and CD81 expression in primary high grade non-muscle invasive bladder cancer: identification of novel biomarkers for bladder cancer using antibody microarray. PLoS One. 2015; 10:e0125405. PMID: 25915404.
Article7. Levy S, Todd SC, Maecker HT. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998; 16:89–109. PMID: 9597125.
Article8. Vences-Catalán F, Duault C, Kuo CC, Rajapaksa R, Levy R, Levy S. CD81 as a tumor target. Biochem Soc Trans. 2017; 45:531–535. PMID: 28408492.
Article9. Vences-Catalán F, Rajapaksa R, Srivastava MK, Marabelle A, Kuo CC, Levy R, et al. Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res. 2015; 75:4517–4526. PMID: 26329536.
Article10. Vences-Catalán F, Rajapaksa R, Srivastava MK, Marabelle A, Kuo CC, Levy R, et al. Tetraspanin CD81, a modulator of immune suppression in cancer and metastasis. Oncoimmunology. 2015; 5:e1120399. PMID: 27467918.
Article11. Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005; 5:136–148. PMID: 15688041.
Article12. Mittelbrunn M, Yáñez-Mó M, Sancho D, Ursa A, Sánchez-Madrid F. Cutting edge: dynamic redistribution of tetraspanin CD81 at the central zone of the immune synapse in both T lymphocytes and APC. J Immunol. 2002; 169:6691–6695. PMID: 12471100.
Article13. Mazzocca A, Liotta F, Carloni V. Tetraspanin CD81-regulated cell motility plays a critical role in intrahepatic metastasis of hepatocellular carcinoma. Gastroenterology. 2008; 135:244–256.e1. PMID: 18466772.
Article14. Carloni V, Mazzocca A, Ravichandran KS. Tetraspanin CD81 is linked to ERK/MAPKinase signaling by Shc in liver tumor cells. Oncogene. 2004; 23:1566–1574. PMID: 14676841.
Article15. Lafleur MA, Xu D, Hemler ME. Tetraspanin proteins regulate membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis. Mol Biol Cell. 2009; 20:2030–2040. PMID: 19211836.
Article16. Hong IK, Byun HJ, Lee J, Jin YJ, Wang SJ, Jeoung DI, et al. The tetraspanin CD81 protein increases melanoma cell motility by up-regulating metalloproteinase MT1-MMP expression through the pro-oncogenic Akt-dependent Sp1 activation signaling pathways. J Biol Chem. 2014; 289:15691–15704. PMID: 24733393.
Article17. Zhang N, Zuo L, Zheng H, Li G, Hu X. Increased expression of CD81 in breast cancer tissue is associated with reduced patient prognosis and increased cell migration and proliferation in MDA-MB-231 and MDA-MB-435S human breast cancer cell lines in vitro. Med Sci Monit. 2018; 24:5739–5747. PMID: 30117494.
Article18. Tohami T, Drucker L, Shapiro H, Radnay J, Lishner M. Overexpression of tetraspanins affects multiple myeloma cell survival and invasive potential. FASEB J. 2007; 21:691–699. PMID: 17210782.
Article19. Yoo TH, Ryu BK, Lee MG, Chi SG. CD81 is a candidate tumor suppressor gene in human gastric cancer. Cell Oncol (Dordr). 2013; 36:141–153. PMID: 23264205.
Article20. White A, Lamb PW, Barrett JC. Frequent downregulation of the KAI1(CD82) metastasis suppressor protein in human cancer cell lines. Oncogene. 1998; 16:3143–3149. PMID: 9671393.
Article21. Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007; 82:1375–1381. PMID: 17709402.
Article22. Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007; 15:2223–2268. PMID: 17275314.
Article23. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002; 2:161–174. PMID: 11990853.
Article24. Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, et al. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993; 53:5365–5369. PMID: 8221672.25. Kanayama H, Yokota K, Kurokawa Y, Murakami Y, Nishitani M, Kagawa S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer. 1998; 82:1359–1366. PMID: 9529029.
Article26. Durkan GC, Nutt JE, Marsh C, Rajjayabun PH, Robinson MC, Neal DE, et al. Alteration in urinary matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio predicts recurrence in nonmuscle-invasive bladder cancer. Clin Cancer Res. 2003; 9:2576–2582. PMID: 12855633.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- STAT3 and ERK Signaling Pathways Are Implicated in the Invasion Activity by Oncostatin M through Induction of Matrix Metalloproteinases 2 and 9
- Effects of Epigallocatechin Gallate on Adhesion, Invasion and Matrix Metalloproteinase Activity in MDA-MB-231 Human Breast Cancer Cells
- The edible ethanol extract of Rosa hybrida suppresses colon cancer progression by inhibiting the proliferation-cell signaling-metastasis axis
- Collagen-induced Activation of MMPs ( Membrane -type Matrix Metalloproteinase and Matrix Metalloproteinase-2) in ovarian cancer cell lines in Vitro
- Expression of Matrix Metalloproteinases and Its Inhibitor in Gastric Adenocarcinoma