Korean J Physiol Pharmacol.
2019 Sep;23(5):345-356. 10.4196/kjpp.2019.23.5.345.
Docosahexaenoic acid reduces adenosine triphosphate-induced calcium influx via inhibition of store-operated calcium channels and enhances baseline endothelial nitric oxide synthase phosphorylation in human endothelial cells
- Affiliations
-
- 1Department of Physiology, Medical Faculty Carl Gustav Carus, Technical University Dresden, Dresden 01307, Germany. thomtbk5@gmail.com
- 2Department of Basic Sciences in Medicine and Pharmacy, School of Medicine and Pharmacy, Vietnam National University, Hanoi 100000, Vietnam.
- 3Faculty of Biology, VNU University of Science, Hanoi 100000, Vietnam.
- 4Dinh Tien Hoang Institute of Medicine, Hanoi 100000, Vietnam.
- KMID: 2455811
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.345
Abstract
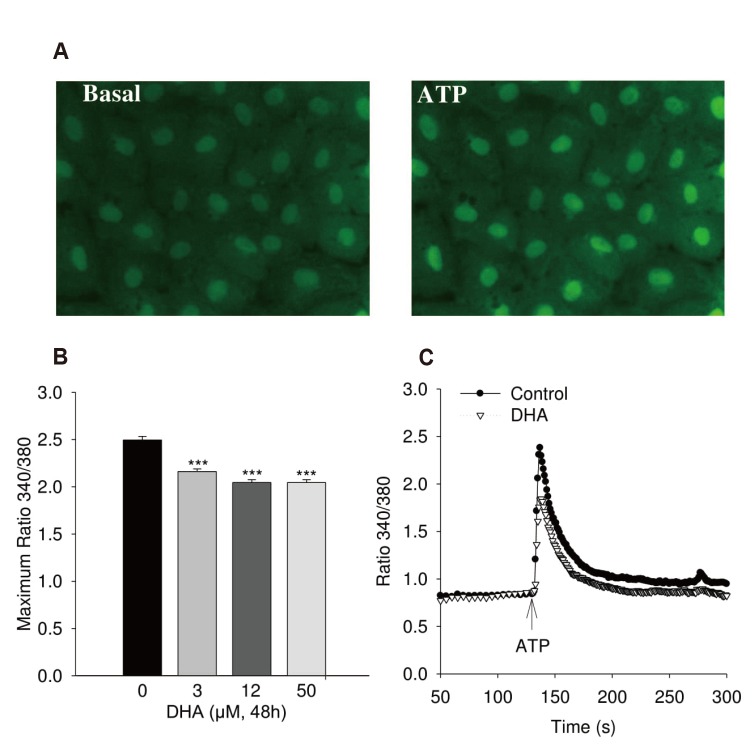
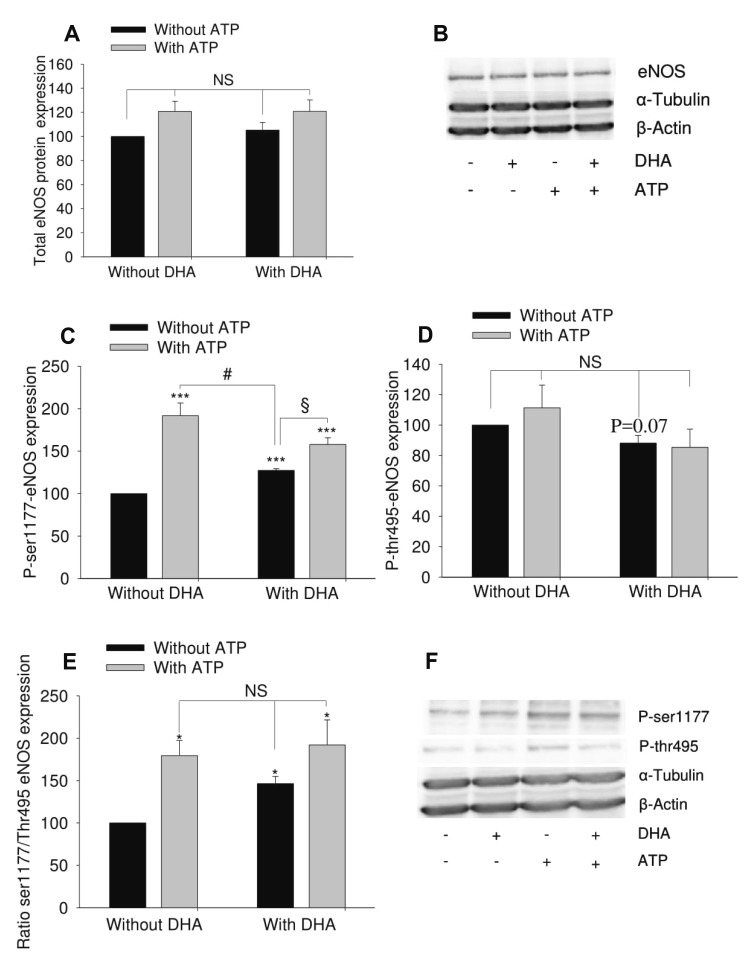
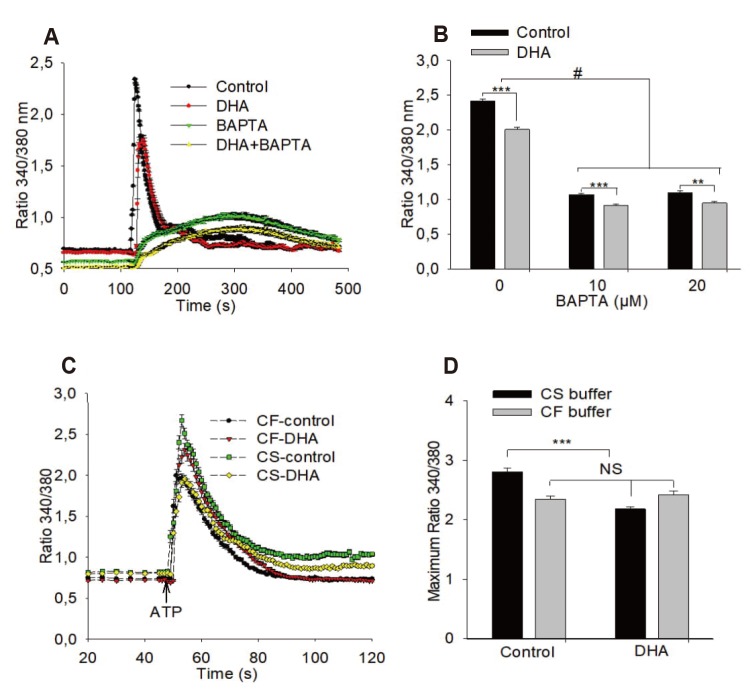
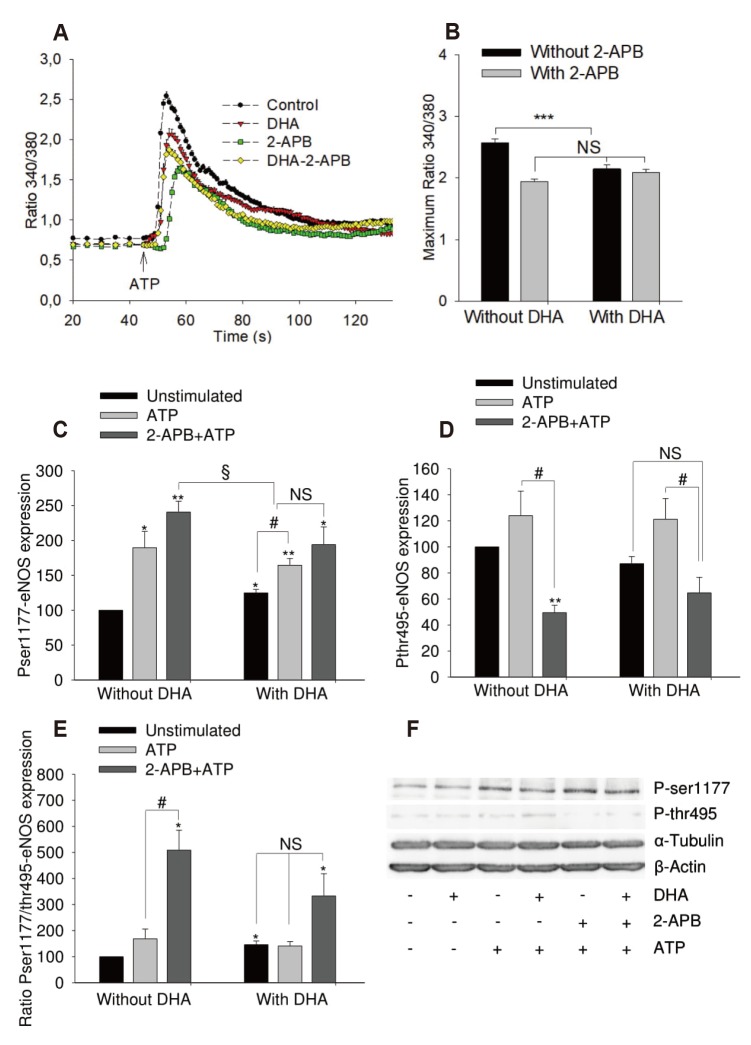
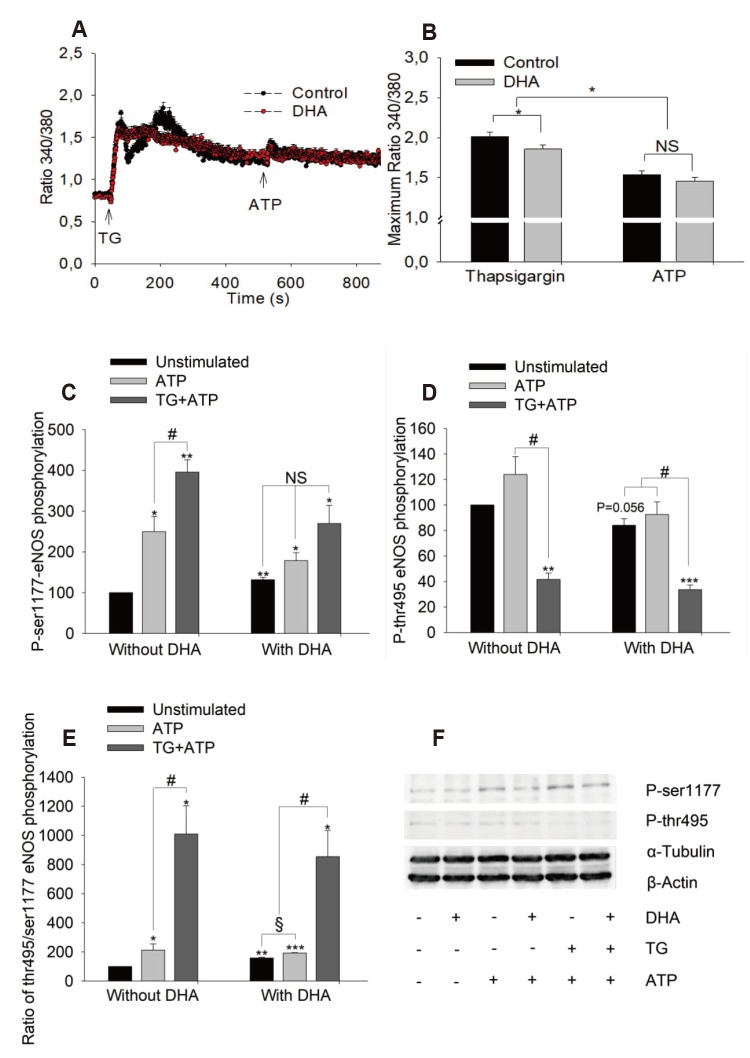
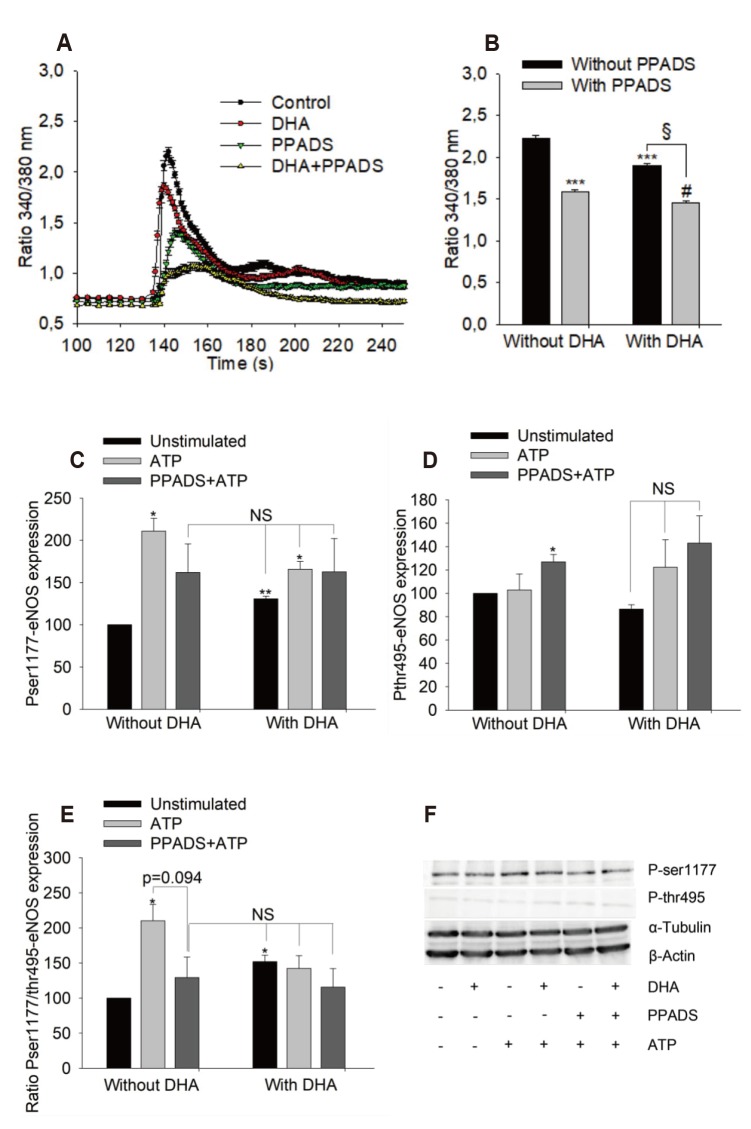
- Docosahexaenoic acid (DHA), an omega-3-fatty acid, modulates multiple cellular functions. In this study, we addressed the effects of DHA on human umbilical vein endothelial cell calcium transient and endothelial nitric oxide synthase (eNOS) phosphorylation under control and adenosine triphosphate (ATP, 100 µM) stimulated conditions. Cells were treated for 48 h with DHA concentrations from 3 to 50 µM. Calcium transient was measured using the fluorescent dye Fura-2-AM and eNOS phosphorylation was addressed by western blot. DHA dose-dependently reduced the ATP stimulated Ca²âº-transient. This effect was preserved in the presence of BAPTA (10 and 20 µM) which chelated the intracellular calcium, but eliminated after withdrawal of extracellular calcium, application of 2-aminoethoxy-diphenylborane (75 µM) to inhibit store-operated calcium channel or thapsigargin (2 µM) to delete calcium store. In addition, DHA (12 µM) increased ser1177/thr495 phosphorylation of eNOS under baseline conditions but had no significant effect on this ratio under conditions of ATP stimulation. In conclusion, DHA dose-dependently inhibited the ATP-induced calcium transient, probably via store-operated calcium channels. Furthermore, DHA changed eNOS phosphorylation suggesting activation of the enzyme. Hence, DHA may shift the regulation of eNOS away from a Ca²âº activated mode to a preferentially controlled phosphorylation mode.
Keyword
MeSH Terms
Figure
Reference
-
1. Holub DJ, Holub BJ. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol Cell Biochem. 2004; 263:217–225.
Article2. Kris-Etherton PM, Harris WS, Appel L. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002; 106:2747–2757. PMID: 12438303.
Article3. Heller AR, Stehr SN, Koch T. Omega 3 fatty acids in clinical nutrition. New York: Nova Science Publishers;2005. p. 108.4. Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006; 83(6 Suppl):1520S–1525S. PMID: 16841862.
Article5. Vitelli MR, Filippelli A, Rinaldi B, Rossi S, Palazzo E, Rossi F, Berrino L. Effects of docosahexaenoic acid on [Ca2+]i increase induced by doxorubicin in ventricular rat cardiomyocytes. Life Sci. 2002; 71:1905–1916. PMID: 12175705.6. Rinaldi B, Di Pierro P, Vitelli MR, D'Amico M, Berrino L, Rossi F, Filippelli A. Effects of docosahexaenoic acid on calcium pathway in adult rat cardiomyocytes. Life Sci. 2002; 71:993–1004. PMID: 12088759.
Article7. Hirafuji M, Ebihara T, Kawahara F, Hamaue N, Endo T, Minami M. Inhibition by docosahexaenoic acid of receptor-mediated Ca2+ influx in rat vascular smooth muscle cells stimulated with 5-hydroxytryptamine. Eur J Pharmacol. 2001; 427:195–201. PMID: 11567649.8. Kuroda R, Hirata K, Kawashima S, Yokoyama M. Unsaturated free fatty acids inhibit Ca2+ mobilization and NO release in endothelial cells. Kobe J Med Sci. 2001; 47:211–219. PMID: 11781499.9. Ye S, Tan L, Ma J, Shi Q, Li J. Polyunsaturated docosahexaenoic acid suppresses oxidative stress induced endothelial cell calcium influx by altering lipid composition in membrane caveolar rafts. Prostaglandins Leukot Essent Fatty Acids. 2010; 83:37–43. PMID: 20206488.
Article10. Kawai Y, Yokoyama Y, Kaidoh M, Ohhashi T. Shear stress-induced ATP-mediated endothelial constitutive nitric oxide synthase expression in human lymphatic endothelial cells. Am J Physiol Cell Physiol. 2010; 298:C647–C655. PMID: 20042732.
Article11. Wilson HL, Varcoe RW, Stokes L, Holland KL, Francis SE, Dower SK, Surprenant A, Crossman DC. P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. Br J Pharmacol. 2007; 151:115–127. PMID: 17351655.
Article12. Thom VT, Wendel M, Deussen A. Regulation of ecto-5′-nucleotidase by docosahexaenoic acid in human endothelial cells. Cell Physiol Biochem. 2013; 32:355–366. PMID: 23988425.
Article13. Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999; 79:703–761. PMID: 10390517.
Article14. Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S. P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J Clin Invest. 2015; 125:3077–3086. PMID: 26168216.15. Burnstock G, Knight GE. Cell culture: complications due to mechanical release of ATP and activation of purinoceptors. Cell Tissue Res. 2017; 370:1–11. PMID: 28434079.
Article16. Tran QK, Ohashi K, Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res. 2000; 48:13–22. PMID: 11033104.
Article17. Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001; 88:E68–E75. PMID: 11397791.
Article18. Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol. 2011; 3:pii:a003970.
Article19. Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015; 95:1383–1436. PMID: 26400989.
Article20. Deussen A, Bading B, Kelm M, Schrader J. Formation and salvage of adenosine by macrovascular endothelial cells. Am J Physiol. 1993; 264(3 Pt 2):H692–H700. PMID: 8456972.
Article21. Sergeeva M, Strokin M, Wang H, Ubl JJ, Reiser G. Arachidonic acid and docosahexaenoic acid suppress thrombin-evoked Ca2+ response in rat astrocytes by endogenous arachidonic acid liberation. J Neurochem. 2002; 82:1252–1261. PMID: 12358772.22. Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000; 279:H285–H292. PMID: 10899068.23. Marrelli SP. TRPV4 channels contribute to ATP-stimulated calcium influx in endothelial cells. FASEB J. 2008; 22(1 suppl 1):964.31.
Article24. Ambudkar IS. TRPC1: a core component of store-operated calcium channels. Biochem Soc Trans. 2007; 35(Pt 1):96–100. PMID: 17233611.
Article25. Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart. 2001; 85:342–350. PMID: 11179281.26. Peluso AA, Bertelsen JB, Andersen K, Mortsensen TP, Hansen PB, Sumners C, Bader M, Santos RA, Steckelings UM. Identification of protein phosphatase involvement in the AT2 receptor-induced activation of endothelial nitric oxide synthase. Clin Sci (Lond). 2018; 132:777–790. PMID: 29540539.27. Sheng JZ, Arshad F, Braun JE, Braun AP. Estrogen and the Ca2+-mobilizing agonist ATP evoke acute NO synthesis via distinct pathways in an individual human vascular endothelium-derived cell. Am J Physiol Cell Physiol. 2008; 294:C1531–C1541. PMID: 18367584.28. da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation. 2009; 119:871–879. PMID: 19188511.
Article29. Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010; 459:793–806. PMID: 20012875.
Article30. Stebbins CL, Stice JP, Hart CM, Mbai FN, Knowlton AA. Effects of dietary decosahexaenoic acid (DHA) on eNOS in human coronary artery endothelial cells. J Cardiovasc Pharmacol Ther. 2008; 13:261–268. PMID: 18682551.
Article31. Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O'Connell TD, Wang D. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 2011; 123:584–593. PMID: 21282499.
Article32. Chao CY, Lii CK, Ye SY, Li CC, Lu CY, Lin AH, Liu KL, Chen HW. Docosahexaenoic acid inhibits vascular endothelial growth factor (VEGF)-induced cell migration via the GPR120/PP2A/ERK1/2/eNOS signaling pathway in human umbilical vein endothelial cells. J Agric Food Chem. 2014; 62:4152–4158. PMID: 24734983.
Article33. Yamagata K. Docosahexaenoic acid regulates vascular endothelial cell function and prevents cardiovascular disease. Lipids Health Dis. 2017; 16:118. PMID: 28619112.
Article34. Nuno DW, Coppey LJ, Yorek MA, Lamping KG. Dietary fats modify vascular fat composition, eNOS localization within lipid rafts and vascular function in obesity. Physiol Rep. 2018; 6:e13820. PMID: 30105819.
Article35. Li Q, Zhang Q, Wang M, Liu F, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys. 2007; 466:250–259. PMID: 17662956.
Article36. Gousset-Dupont A, Robert V, Grynberg A, Lacour B, Tardivel S. The effect of n-3 PUFA on eNOS activity and expression in Ea hy 926 cells. Prostaglandins Leukot Essent Fatty Acids. 2007; 76:131–139. PMID: 17229561.
Article37. Allam-Ndoul B, Guénard F, Barbier O, Vohl MC. Effect of different concentrations of omega-3 fatty acids on stimulated THP-1 macrophages. Genes Nutr. 2017; 12:7. PMID: 28250850.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vascular Smooth Muscle Adenosine Triphosphate (ATP)-Sensitive Potassium Channel and Anesthetics
- Tat-Mediated p66shc Transduction Decreased Phosphorylation of Endothelial Nitric Oxide Synthase in Endothelial Cells
- Inhibition of Store-Operated Calcium Entry Protects Endothelial Progenitor Cells from Hâ‚‚Oâ‚‚-Induced Apoptosis
- The Relaxant Effect of Propofol on Isolated Rat Intrapulmonary Arteries
- Mechanism of L-NAME-resistant endothelium-dependent relaxation induced by acetylcholine in rabbit renal artery