Cancer Res Treat.
2016 Apr;48(2):473-482. 10.4143/crt.2015.116.
Overexpression of SOX2 Is Associated with Better Overall Survival in Squamous Cell Lung Cancer Patients Treated with Adjuvant Radiotherapy
- Affiliations
-
- 1Department of Pharmacology, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. ybkim3@yuhs.ac
- 3Yonsei Song-Dang Institute for Cancer Research, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2454323
- DOI: http://doi.org/10.4143/crt.2015.116
Abstract
- PURPOSE
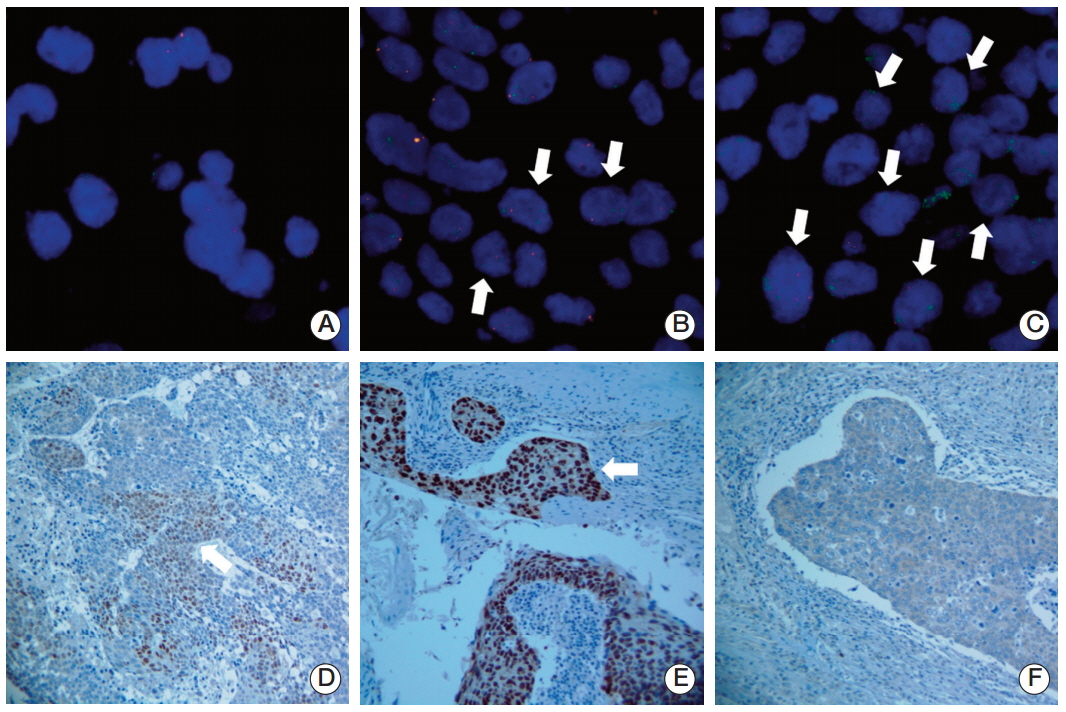
The purpose of this study is to investigate the prognostic significance of SOX2 gene amplification and expression in patients with American Joint Committee on Cancer stage III lung squamous cell carcinoma (SCC) who underwent surgery followed by adjuvant radiotherapy.
MATERIALS AND METHODS
Pathological specimens were obtained from 33 patients with stage III lung SCC treated with surgery followed by adjuvant radiotherapy between 1996 and 2008. SOX2 gene amplification and protein expression were analyzed using fluorescent in situ hybridization and immunohistochemistry, respectively. Patients were divided into two groups according to their SOX2 gene amplification and protein expression status. Kaplan-Meier estimates and a Cox proportional hazards model were used to identify the prognostic factors affecting patient survival.
RESULTS
The median follow-up period for surviving patients was 58 months (range, 5 to 102 months). SOX2 gene amplification was observed in 22 patients and protein overexpression in 26 patients. SOX2 overexpression showed significant association with SOX2 gene amplification (p=0.002). In multivariate analysis, SOX2 overexpression was a significant prognostic factor for overall survival (OS) (hazard ratios [HR], 0.1; 95% confidence interval [CI], 0.002 to 0.5; p=0.005) and disease-free survival (DFS) (HR, 0.15; 95% CI, 0.04 to 0.65; p=0.01). Age (HR, 0.33; 95% CI, 0.11 to 0.98; p=0.046) and total radiation dose (HR, 0.13; 95% CI, 0.02 to 0.7; p=0.02) were the independent prognostic factors for OS and DFS. Patients with SOX2 amplification did not show a longer OS (p=0.95) and DFS (p=0.48).
CONCLUSION
Our data suggested that SOX2 overexpression could be used as a positive prognostic factor in patients with stage III lung SCC receiving adjuvant radiotherapy.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012; 44:25–31.
Article2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article3. Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009; 41:1238–42.
Article4. Sutherland KD, Berns A. Cell of origin of lung cancer. Mol Oncol. 2010; 4:397–403.
Article5. Long KB, Hornick JL. SOX2 is highly expressed in squamous cell carcinomas of the gastrointestinal tract. Hum Pathol. 2009; 40:1768–73.
Article6. Yuan P, Kadara H, Behrens C, Tang X, Woods D, Solis LM, et al. Sex determining region Y-Box 2 (SOX2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLoS One. 2010; 5:e9112.
Article7. Velcheti V, Schalper K, Yao X, Cheng H, Kocoglu M, Dhodapkar K, et al. High SOX2 levels predict better outcome in non-small cell lung carcinomas. PLoS One. 2013; 8:e61427.
Article8. Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, Maier S, et al. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol. 2011; 24:944–53.9. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC cancer staging manual. 6th ed. New York: Springer;2002.10. Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol. 2008; 317:296–309.
Article11. Hussenet T, du Manoir S. SOX2 in squamous cell carcinoma: amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle. 2010; 9:1480–6.
Article12. Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, et al. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013; 25:1264–71.
Article13. Singh S, Trevino J, Bora-Singhal N, Coppola D, Haura E, Altiok S, et al. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol Cancer. 2012; 11:73.
Article14. Chen S, Xu Y, Chen Y, Li X, Mou W, Wang L, et al. SOX2 gene regulates the transcriptional network of oncogenes and affects tumorigenesis of human lung cancer cells. PLoS One. 2012; 7:e36326.
Article15. Chen S, Li X, Lu D, Xu Y, Mou W, Wang L, et al. SOX2 regulates apoptosis through MAP4K4-survivin signaling pathway in human lung cancer cells. Carcinogenesis. 2014; 35:613–23.
Article16. Wu F, Zhang J, Wang P, Ye X, Jung K, Bone KM, et al. Identification of two novel phenotypically distinct breast cancer cell subsets based on Sox2 transcription activity. Cell Signal. 2012; 24:1989–98.
Article17. Lin F, Lin P, Zhao D, Chen Y, Xiao L, Qin W, et al. Sox2 targets cyclinE, p27 and survivin to regulate androgen-independent human prostate cancer cell proliferation and apoptosis. Cell Prolif. 2012; 45:207–16.18. Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem. 2008; 283:17969–78.
Article19. Fang X, Yoon JG, Li L, Tsai YS, Zheng S, Hood L, et al. Landscape of the SOX2 protein-protein interactome. Proteomics. 2011; 11:921–34.
Article20. Niu Y, Zhang X, Zheng Y, Zhang R. XRCC1 deficiency increased the DNA damage induced by gamma-ray in HepG2 cell: involvement of DSB repair and cell cycle arrest. Environ Toxicol Pharmacol. 2013; 36:311–9.21. Chang HW, Kim SY, Yi SL, Son SH, Song DY, Moon SY. Expression of Ku80 correlates with sensitivities to radiation in cancer cell lines of the head and neck. Oral Oncol. 2006; 42:979–86.
Article22. Ayene IS, Ford LP, Koch CJ. Ku protein targeting by Ku70 small interfering RNA enhances human cancer cell response to topoisomerase II inhibitor and gamma radiation. Mol Cancer Ther. 2005; 4:529–36.23. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006; 444:756–60.
Article24. Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009; 458:780–3.
Article25. Wang X, Ji X, Chen J, Yan D, Zhang Z, Wang Q, et al. SOX2 enhances the migration and invasion of ovarian cancer cells via Src kinase. PLoS One. 2014; 9:e99594.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adjuvant Chemotherapy for Completely Resected Non-Small Cell Lung Cancer
- Clinical Features of the Lung Cancer Patients Who Were Seen in Kosin University Gospel Hospital from 1994 to 1998
- Implantation Metastasis of Lung Cancer to Chest Wall after Percutaneous Fine-Needle Aspiration Biopsy
- Definitive Radiotherapy of Non-Small Cell Lung Cancer
- Radiation Therapy in Non-Small Cell LUNg Cancer