Tuberc Respir Dis.
2019 Apr;82(2):158-165. 10.4046/trd.2018.0044.
Potential Therapeutic Strategy in Chronic Obstructive Pulmonary Disease Using Pioglitazone-Augmented Wharton's Jelly-Derived Mesenchymal Stem Cells
- Affiliations
-
- 1Asan Institute for Life Sciences, Seoul, Korea. ymoh55@amc.seoul.kr
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Busan, Korea.
- 3Department of Pulmonary and Critical Care Medicine, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pulmonary and Critical Care Medicine, Asan Medical Center, Seoul, Korea.
- KMID: 2453986
- DOI: http://doi.org/10.4046/trd.2018.0044
Abstract
- BACKGROUND
A recent study reported that mesenchymal stem cells possess potential cellular therapeutic properties for treating patients with chronic obstructive pulmonary disease, which is characterized by emphysema. We examined the potential therapeutic effect of Wharton's Jelly-derived mesenchymal stem cells (WJMSCs), following pretreatment with pioglitazone, in lung regeneration mouse emphysema models.
METHODS
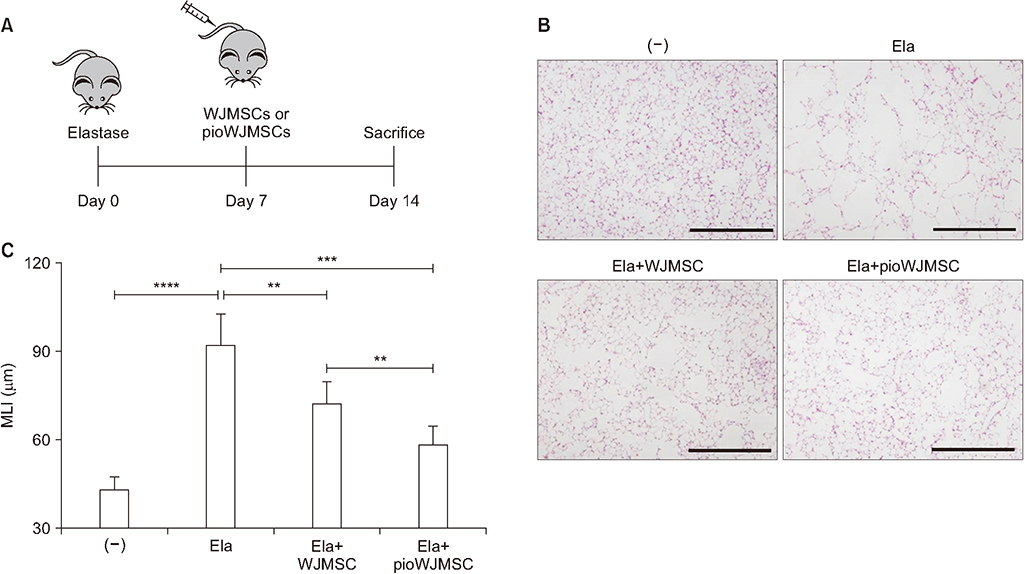
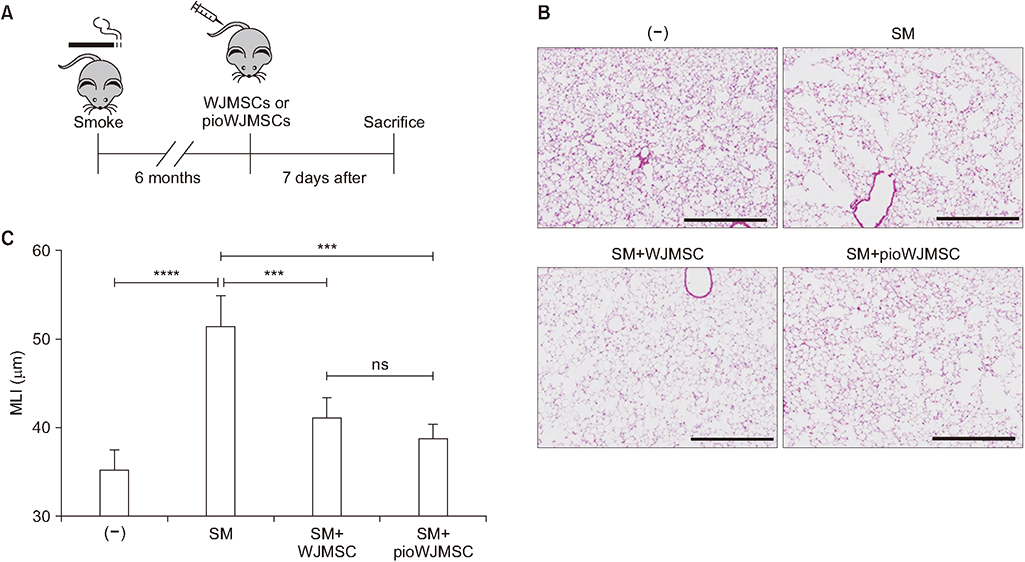
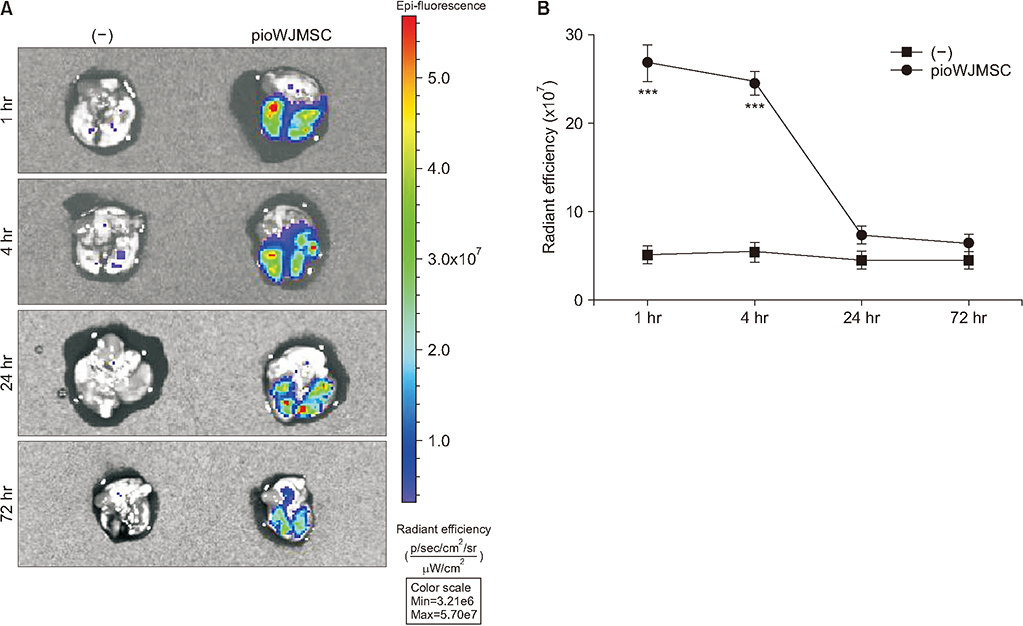
We used two mouse emphysema models, an elastase-induced model and a cigarette smoke-induced model. We intravenously injected WJMSCs (1×104/mouse) to mice, pretreated or not, with pioglitazone for 7 days. We measured the emphysema severity by mean linear intercepts (MLI) analysis using lung histology.
RESULTS
Pioglitazone pretreated WJMSCs (pioWJMSCs) were associated with greater lung regeneration than non-augmented WJMSCs in the two mouse emphysema models. In the elastase-induced emphysema model, the MLIs were 59.02±2.42 µm (n=6), 72.80±2.87 µm (n=6), for pioWJMSCs injected mice, and non-augmented WJMSCs injected mice, respectively (p<0.01). Both pioWJMSCs and non-augmented WJMSCs showed regenerative effects in the cigarette smoke emphysema model (MLIs were 41.25±0.98 [n=6] for WJMSCs and38.97±0.61 µm [n=6] for pioWJMSCs) compared to smoking control mice (51.65±1.36 µm, n=6). The mean improvement of MLI appeared numerically better in pioWJMSCs than in non-augmented WJMSCs injected mice, but the difference did not reach the level of statistical significance (p=0.071).
CONCLUSION
PioWJMSCs may produce greater lung regeneration, compared to non-augmented WJMSCs, in a mouse emphysema model.
Keyword
MeSH Terms
Figure
Reference
-
1. Murphy TF, Sethi S. Chronic obstructive pulmonary disease: role of bacteria and guide to antibacterial selection in the older patient. Drugs Aging. 2002; 19:761–775.2. Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–365.3. Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet. 2004; 364:613–620.
Article4. Agusti AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003; 21:347–360.
Article5. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004; 364:709–721.
Article6. MacNee W, Tuder RM. New paradigms in the pathogenesis of chronic obstructive pulmonary disease I. Proc Am Thorac Soc. 2009; 6:527–531.
Article7. Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007; 87:1047–1082.
Article8. Cho GS, Fernandez L, Kwon C. Regenerative medicine for the heart: perspectives on stem-cell therapy. Antioxid Redox Signal. 2014; 21:2018–2031.
Article9. Donega V, Nijboer CH, van Velthoven CT, Youssef SA, de Bruin A, van Bel F, et al. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res. 2015; 78:520–526.
Article10. Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005; 11:3431–3440.
Article11. Zhu Y, Chen X, Yang X, Ei-Hashash A. Stem cells in lung repair and regeneration: current applications and future promise. J Cell Physiol. 2018; 233:6414–6424.
Article12. Matthay MA, Goolaerts A, Howard JP, Lee JW. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010; 38:10 Suppl. S569–S573.
Article13. Longhini-Dos-Santos N, Barbosa-de-Oliveira VA, Kozma RH, Faria CA, Stessuk T, Frei F, et al. Cell therapy with bone marrow mononuclear cells in elastase-induced pulmonary emphysema. Stem Cell Rev. 2013; 9:210–218.
Article14. Huh JW, Kim SY, Lee JH, Lee JS, Van Ta Q, Kim M, et al. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011; 301:L255–L266.
Article15. Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011; 19:196–203.
Article16. Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013; 14:11692–11712.17. Hammam OA, Elkhafif N, Attia YM, Mansour MT, Elmazar MM, Abdelsalam RM, et al. Wharton's jelly-derived mesenchymal stem cells combined with praziquantel as a potential therapy for Schistosoma mansoni-induced liver fibrosis. Sci Rep. 2016; 6:21005.
Article18. Tamura M, Kawabata A, Ohta N, Uppalapati L, Becker KG, Troyer D. Wharton's jelly stem cells as agents for cancer therapy. Open Tissue Eng Regen Med J. 2011; 4:39–47.
Article19. Scheers I, Lombard C, Najimi M, Sokal EM. Cell therapy for the treatment of metabolic liver disease: an update on the umbilical cord derived stem cells candidates. Open Tissue Eng Regen Med J. 2011; 4:48–53.20. Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007; 14:828–835.
Article21. Hong Y, Kim YS, Hong SH, Oh YM. Therapeutic effects of adipose-derived stem cells pretreated with pioglitazone in an emphysema mouse model. Exp Mol Med. 2016; 48:e266.
Article22. Kim YS, Kim JY, Huh JW, Lee SW, Choi SJ, Oh YM. The therapeutic effects of optimal dose of mesenchymal stem cells in a murine model of an elastase induced-emphysema. Tuberc Respir Dis. 2015; 78:239–245.
Article23. Thurlbeck WM. Measurement of pulmonary emphysema. Am Rev Respir Dis. 1967; 95:752–764.24. Kim YS, Kim JY, Shin DM, Huh JW, Lee SW, Oh YM. Tracking intravenous adipose-derived mesenchymal stem cells in a model of elastase-induced emphysema. Tuberc Respir Dis. 2014; 77:116–123.
Article25. Kim YS, Kim JY, Cho R, Shin DM, Lee SW, Oh YM. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp Mol Med. 2017; 49:e284.
Article26. Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006; 24:781–792.
Article27. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
Article28. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006; 24:150–154.
Article29. Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res Ther. 2014; 5:53.
Article30. Yoon JH, Roh EY, Shin S, Jung NH, Song EY, Chang JY, et al. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton's jelly. Biomed Res Int. 2013; 2013:428726.
Article31. Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, et al. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008; 26:2865–2874.
Article32. Jyothi Prasanna S, Sowmya Jahnavi V. Wharton's jelly mesenchymal stem cells as off-the-shelf cellular therapeutics: a closer look into their regenerative and immunomodulatory properties. Open Tissue Eng Regen Med J. 2011; 4:28–38.
Article33. Hackett TL, Knight DA, Sin DD. Potential role of stem cells in management of COPD. Int J Chron Obstruct Pulmon Dis. 2010; 5:81–88.34. Yoshida H, Kitaichi T, Urata M, Kurobe H, Kanbara T, Motoki T, et al. Syngeneic bone marrow mononuclear cells improve pulmonary arterial hypertension through vascular endothelial growth factor upregulation. Ann Thorac Surg. 2009; 88:418–424.
Article35. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013; 45:e54.
Article36. Muyal JP, Muyal V, Kotnala S, Kumar D, Bhardwaj H. Therapeutic potential of growth factors in pulmonary emphysematous condition. Lung. 2013; 191:147–163.
Article37. Guan XJ, Song L, Han FF, Cui ZL, Chen X, Guo XJ, et al. Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem. 2013; 114:323–335.
Article38. Morino S, Nakamura T, Toba T, Takahashi M, Kushibiki T, Tabata Y, et al. Fibroblast growth factor-2 induces recovery of pulmonary blood flow in canine emphysema models. Chest. 2005; 128:920–926.
Article39. Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003; 33:919–926.
Article40. Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003; 21:105–110.
Article41. Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, et al. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007; 25:319–331.42. Carlin R, Davis D, Weiss M, Schultz B, Troyer D. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006; 4:8.43. Choudhery MS, Badowski M, Muise A, Harris DT. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy. 2013; 15:330–343.
Article44. Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004; 22:1330–1337.
Article45. Yukawa H, Mizufune S, Mamori C, Kagami Y, Oishi K, Kaji N, et al. Quantum dots for labeling adipose tissue-derived stem cells. Cell Transplant. 2009; 18:591–599.
Article46. Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, et al. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012; 33:2177–2186.
Article47. Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet. 2002; 3:370–379.
Article48. Nicklas JA, Buel E. Development of an Alu-based, real-time PCR method for quantitation of human DNA in forensic samples. J Forensic Sci. 2003; 48:936–944.
Article49. Batzer MA, Gudi VA, Mena JC, Foltz DW, Herrera RJ, Deininger PL. Amplification dynamics of human-specific (HS) Alu family members. Nucleic Acids Res. 1991; 19:3619–3623.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic effects of adipose-derived stem cells pretreated with pioglitazone in an emphysema mouse model
- Mesenchymal Stem Cells from the Wharton's Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential
- Transplantation of Gelatin Microspheres Loaded with Wharton’s Jelly Derived Mesenchymal Stem Cells Facilitates Cartilage Repair in Mice
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- Cardioprotective Effects of Wharton Jelly Derived Mesenchymal Stem Cell Transplantation in a Rodent Model of Myocardial Injury