J Pathol Transl Med.
2019 May;53(3):180-187. 10.4132/jptm.2019.02.08.
Association between p53 Expression and Amount of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. backlila@gmail.com
- 2Asan Center for Cancer Genome Discovery, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2449341
- DOI: http://doi.org/10.4132/jptm.2019.02.08
Abstract
- BACKGROUND
Most triple-negative breast cancers (TNBCs) have a high histologic grade, are associated with high endoplasmic stress, and possess a high frequency of TP53 mutations. TP53 missense mutations lead to the production of mutant p53 protein and usually show high levels of p53 protein expression. Tumor-infiltrating lymphocytes (TILs) accumulate as part of the anti-tumor immune response and have a strong prognostic and predictive significance in TNBC. We aimed to elucidate the association between p53 expression and the amount of TILs in TNBC.
METHODS
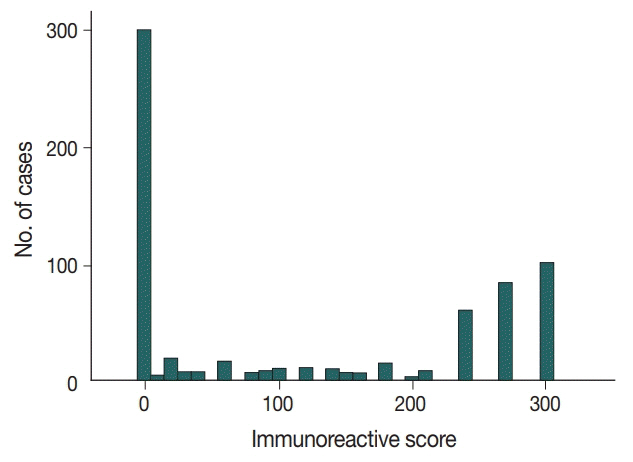
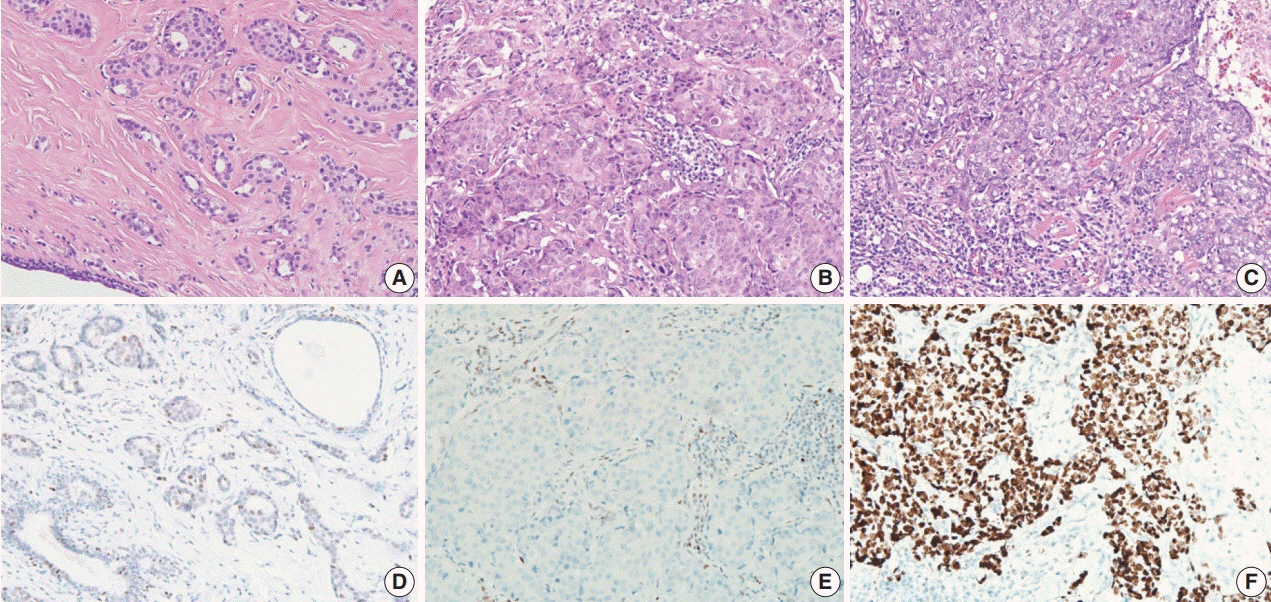
In 678 TNBC patients, we evaluated TIL levels and expression of endoplasmic stress molecules. Immunohistochemical examination of p53 protein expression was categorized into three groups: no, low, and high expression.
RESULTS
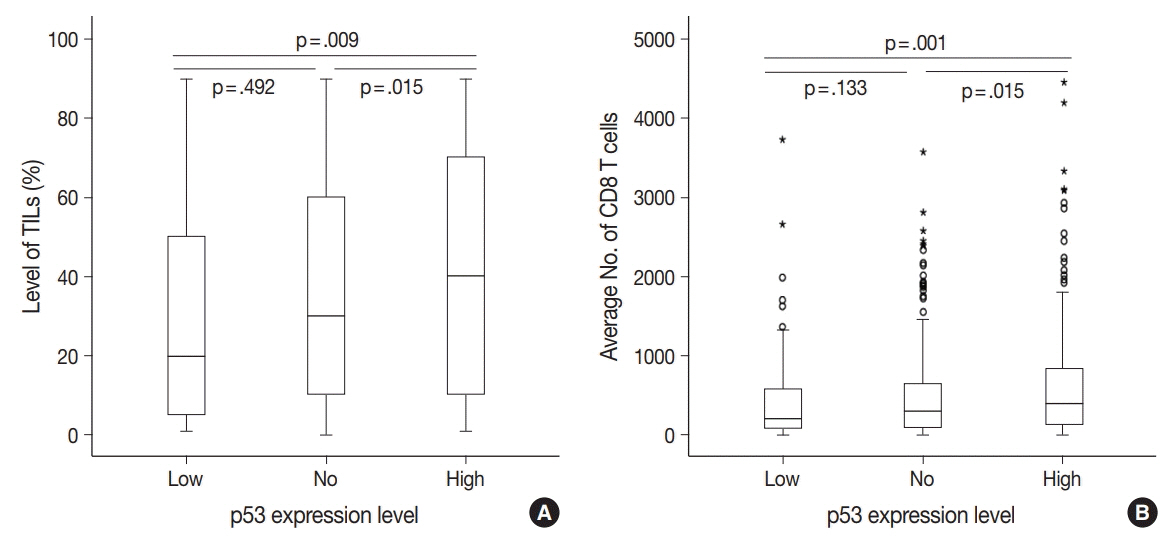
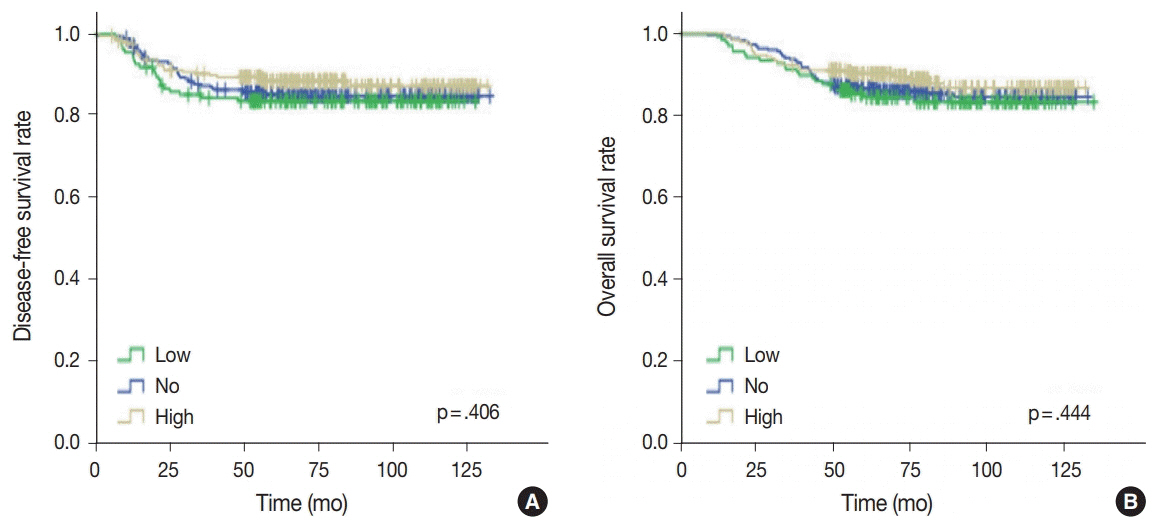
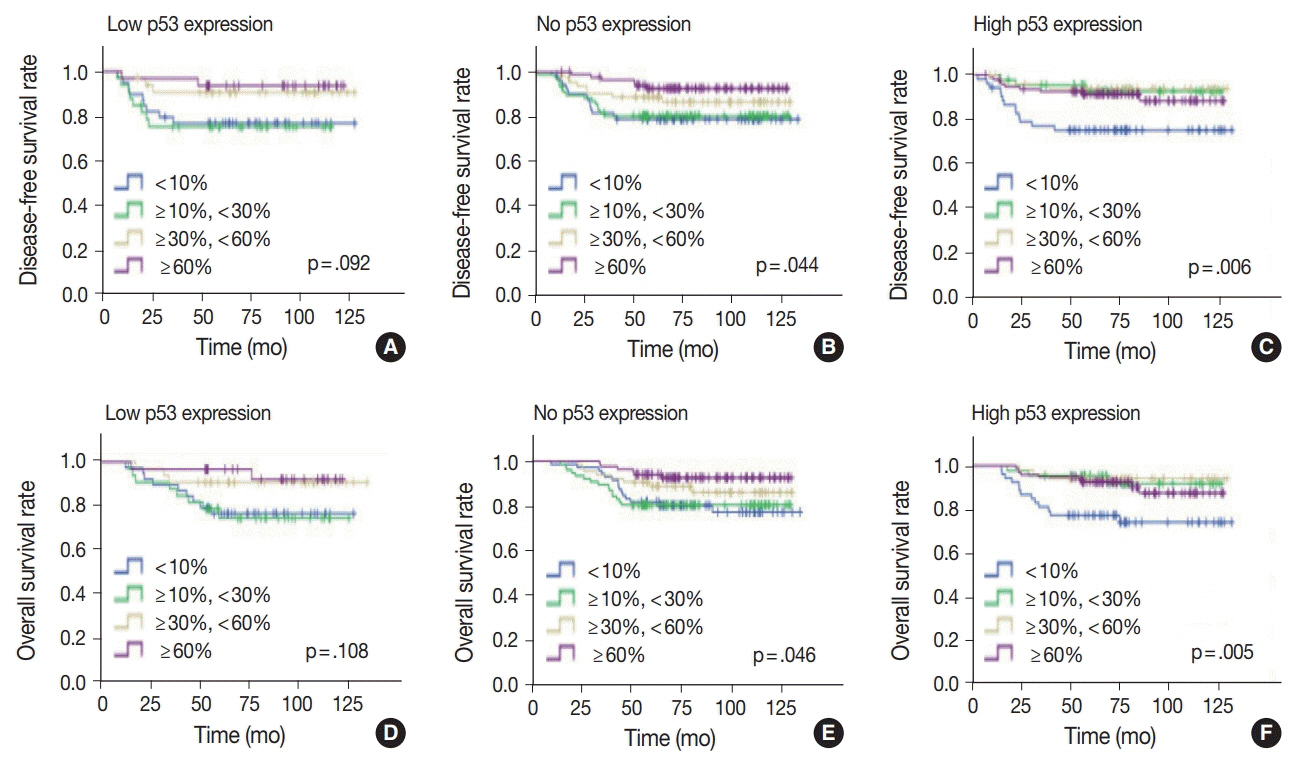
No, low, and high p53 expression was identified in 44.1% (n = 299), 20.1% (n = 136), and 35.8% (n = 243) of patients, respectively. Patients with high p53 expression showed high histologic grade (p < .001), high TIL levels (p = .009), and high expression of endoplasmic reticulum stress-associated molecules (p-eIF2a, p = .013; XBP1, p = .007), compared to patients with low p53 expression. There was no significant difference in disease-free (p = .406) or overall survival rates (p = .444) among the three p53 expression groups.
CONCLUSIONS
High p53 expression is associated with increased expression of endoplasmic reticulum stress molecules and TIL influx.
MeSH Terms
Figure
Reference
-
1. Yamashita N, Kondo M, Zhao S, et al. Picrasidine G decreases viability of MDA-MB 468 EGFR-overexpressing triple-negative breast cancer cells through inhibition of EGFR/STAT3 signaling pathway. Bioorg Med Chem Lett. 2017; 27:2608–12.
Article2. Yadav BS, Chanana P, Jhamb S. Biomarkers in triple negative breast cancer: a review. World J Clin Oncol. 2015; 6:252–63.
Article3. van Rooijen JM, Stutvoet TS, Schroder CP, de Vries EG. Immunotherapeutic options on the horizon in breast cancer treatment. Pharmacol Ther. 2015; 156:90–101.
Article4. Kim JY, Heo SH, Song IH, et al. Activation of the PERK-eIF2alpha pathway is associated with tumor-infiltrating lymphocytes in HER2-positive breast cancer. Anticancer Res. 2016; 36:2705–11.5. Kim YA, Lee HJ, Heo SH, et al. MxA expression is associated with tumor-infiltrating lymphocytes and is a prognostic factor in triplenegative breast cancer. Breast Cancer Res Treat. 2016; 156:597–606.
Article6. Lee HJ, Park IA, Song IH, et al. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. 2016; 69:422–30.
Article7. Lee HJ, Song IH, Park IA, et al. Differential expression of major histocompatibility complex class I in subtypes of breast cancer is associated with estrogen receptor and interferon signaling. Oncotarget. 2016; 7:30119–32.
Article8. Park IA, Heo SH, Song IH, et al. Endoplasmic reticulum stress induces secretion of high-mobility group proteins and is associated with tumor-infiltrating lymphocytes in triple-negative breast cancer. Oncotarget. 2016; 7:59957–64.
Article9. Chen X, Iliopoulos D, Zhang Q, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014; 508:103–7.10. Han CC, Wan FS. New insights into the role of endoplasmic reticulum stress in breast cancer metastasis. J Breast Cancer. 2018; 21:354–62.
Article11. Oros Klein K, Oualkacha K, Lafond MH, Bhatnagar S, Tonin PN, Greenwood CM. Gene coexpression analyses differentiate networks associated with diverse cancers harboring TP53 missense or null mutations. Front Genet. 2016; 7:137.
Article12. Kandioler-Eckersberger D, Ludwig C, Rudas M, et al. TP53 mutation and p53 overexpression for prediction of response to neoadjuvant treatment in breast cancer patients. Clin Cancer Res. 2000; 6:50–6.13. Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009; 9:701–13.
Article14. Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012; 26:1268–86.
Article15. Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013; 15:2–8.
Article16. Yue X, Zhao Y, Xu Y, Zheng M, Feng Z, Hu W. Mutant p53 in cancer: accumulation, gain-of-function, and therapy. J Mol Biol. 2017; 429:1595–606.
Article17. Alsner J, Yilmaz M, Guldberg P, Hansen LL, Overgaard J. Heterogeneity in the clinical phenotype of TP53 mutations in breast cancer patients. Clin Cancer Res. 2000; 6:3923–31.18. Jin MS, Park IA, Kim JY, et al. New insight on the biological role of p53 protein as a tumor suppressor: re-evaluation of its clinical significance in triple-negative breast cancer. Tumour Biol. 2016; 37:11017–24.
Article19. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490:61–70.
Article20. Duffy MJ, Synnott NC, McGowan PM, Crown J, O’Connor D, Gallagher WM. p53 as a target for the treatment of cancer. Cancer Treat Rev. 2014; 40:1153–60.
Article21. Duffy MJ, Synnott NC, Crown J. Mutant p53 as a target for cancer treatment. Eur J Cancer. 2017; 83:258–65.
Article22. Kojima YA, Wang X, Sun H, Compton F, Covinsky M, Zhang S. Reproducible evaluation of tumor-infiltrating lymphocytes (TILs) using the recommendations of International TILs Working Group 2014. Ann Diagn Pathol. 2018; 35:77–9.
Article23. Park IA, Hwang SH, Song IH, et al. Expression of the MHC class II in triple-negative breast cancer is associated with tumor-infiltrating lymphocytes and interferon signaling. PLoS One. 2017; 12:e0182786.
Article24. Adams S, Goldstein LJ, Sparano JA, Demaria S, Badve SS. Tumor infiltrating lymphocytes (TILs) improve prognosis in patients with triple negative breast cancer (TNBC). Oncoimmunology. 2015; 4:e985930.
Article25. Lee HJ, Kim A, Song IH, et al. Cytoplasmic expression of high mobility group B1 (HMGB1) is associated with tumor-infiltrating lymphocytes (TILs) in breast cancer. Pathol Int. 2016; 66:202–9.
Article26. Darb-Esfahani S, Denkert C, Stenzinger A, et al. Role of TP53 mutations in triple negative and HER2-positive breast cancer treated with neoadjuvant anthracycline/taxane-based chemotherapy. Oncotarget. 2016; 7:67686–98.27. Maeda T, Nakanishi Y, Hirotani Y, et al. Immunohistochemical co-expression status of cytokeratin 5/6, androgen receptor, and p53 as prognostic factors of adjuvant chemotherapy for triple negative breast cancer. Med Mol Morphol. 2016; 49:11–21.
Article28. Wu M, Wei W, Xiao X, et al. Expression of SIRT1 is associated with lymph node metastasis and poor prognosis in both operable triplenegative and non-triple-negative breast cancer. Med Oncol. 2012; 29:3240–9.
Article29. Zhang J, Wang Y, Yin Q, Zhang W, Zhang T, Niu Y. An associated classification of triple negative breast cancer: the risk of relapse and the response to chemotherapy. Int J Clin Exp Pathol. 2013; 6:1380–91.30. Biganzoli E, Coradini D, Ambrogi F, et al. p53 status identifies two subgroups of triple-negative breast cancers with distinct biological features. Jpn J Clin Oncol. 2011; 41:172–9.
Article31. Kashiwagi S, Yashiro M, Takashima T, et al. Advantages of adjuvant chemotherapy for patients with triple-negative breast cancer at Stage II: usefulness of prognostic markers E-cadherin and Ki67. Breast Cancer Res. 2011; 13:R122.
Article32. Wang J, Zhang C, Chen K, et al. ERbeta1 inversely correlates with PTEN/PI3K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res Treat. 2015; 152:255–69.33. Lheureux S, Denoyelle C, Ohashi PS, De Bono JS, Mottaghy FM. Molecularly targeted therapies in cancer: a guide for the nuclear medicine physician. Eur J Nucl Med Mol Imaging. 2017; 44(Suppl 1):41–54.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Issues and Clinical Evidence in Tumor-Infiltrating Lymphocytes in Breast Cancer
- Differences in prognosis by p53 expression after neoadjuvant chemotherapy in triple-negative breast cancer
- Prognostic Role and Clinical Association of Tumor-Infiltrating Lymphocyte, Programmed Death Ligand-1 Expression with Neutrophil-Lymphocyte Ratio in Locally Advanced Triple-Negative Breast Cancer
- Expression of Immunoproteasome Subunit LMP7 in Breast Cancer and Its Association with Immune-Related Markers
- p53 Protein Expression in Infiltrating Ductal Carcinoma of the Breast