Ann Surg Treat Res.
2020 Jun;98(6):291-298. 10.4174/astr.2020.98.6.291.
Differences in prognosis by p53 expression after neoadjuvant chemotherapy in triple-negative breast cancer
- Affiliations
-
- 1Department of Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 2Department of Pathology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2502114
- DOI: http://doi.org/10.4174/astr.2020.98.6.291
Abstract
- Purpose
Our previous studies suggested that p53-positive triple-negative breast cancer (TNBC) should be more sensitive to chemotherapy than p53-negative TNBC. The aim of this study was to determine whether p53 expression in TNBC could predict response to neoadjuvant chemotherapy and the resulting prognosis.
Methods
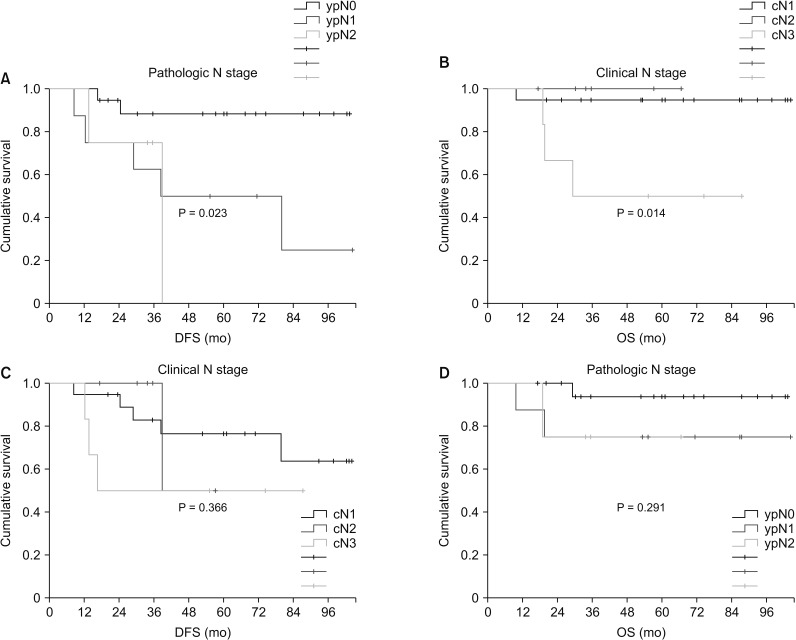
From January 2009 to December 2017, TNBC patients who underwent neoadjuvant chemotherapy were reviewed, including a total of 31 TNBC patients who had clinical lymph node metastasis. The status of p53 expression in patients before and after chemotherapy was evaluated.
Results
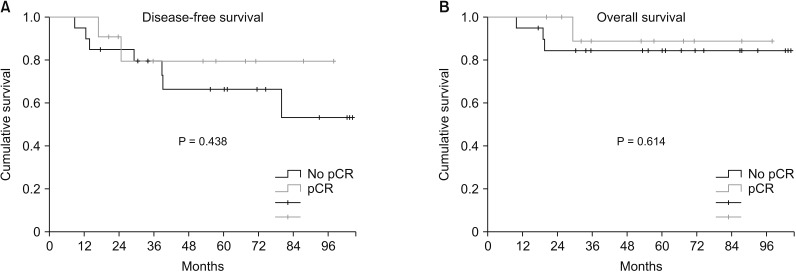
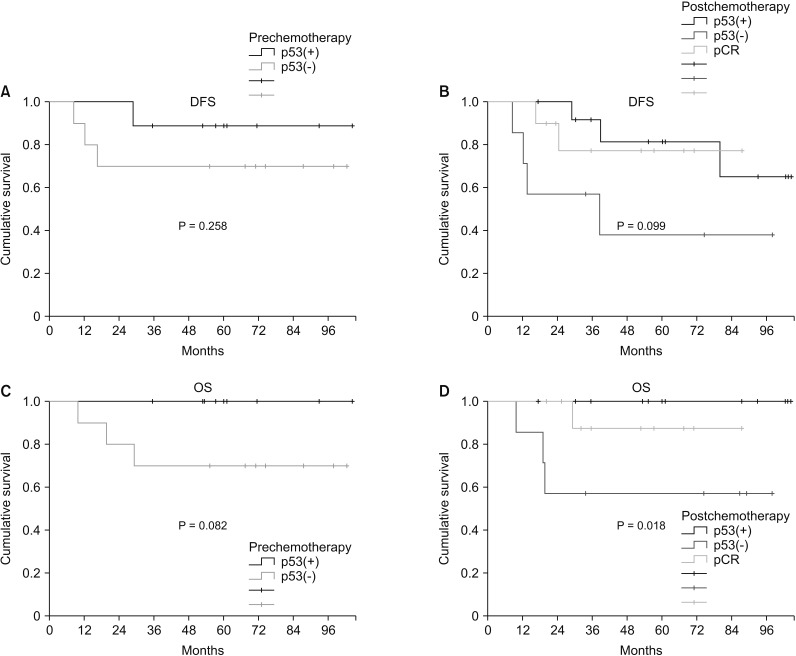
Two patients (22.2%, 2 of 9) achieved pCR in p53(+) TNBC and 4 patients (50%, 5 of 10) achieved pCR in p53(-) TNBC. There was no correlation between pCR rate and p53 expression (P = 0.350). Based on prechemotherapy p53 expression, there was no significant difference in disease-free survival (DFS) between p53(+) TNBC and p53(-) TNBC (P = 0.335). However, after chemotherapy, p53(+) TNBC had shown higher DFS than p53(-) TBNC (P = 0.099). Based on prechemotherapy p53 expression, p53(+) TNBC had better overall survival (OS) than p53(-) TNBC, but the difference was not statistically significant (P = 0.082). After chemotherapy, p53(+) TNBC showed significantly better OS than p53(-) TNBC (P = 0.018).
Conclusion
Immunohistochemically detected p53 expression in TNBC could not predict the response to neoadjuvant chemotherapy. However, p53(+) TNBC had a better OS than p53(-) TNBC in patients who underwent neoadjuvant chemotherapy.
Keyword
Figure
Reference
-
1. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010; 363:1938–1948. PMID: 21067385.2. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13:4429–4434. PMID: 17671126.3. Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–1281. PMID: 18250347.4. von Minckwitz G, Martin M. Neoadjuvant treatments for triple-negative breast cancer (TNBC). Ann Oncol. 2012; 23 Suppl 6:vi35–vi39. PMID: 23012300.5. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804. PMID: 22508812.6. National Comprehensive Cancer Network. NCCN Guidelines Version 3. 2018 Breast Cancer [Internet]. Fort Wathington (PA): National Comprehensive Cancer Network;c2018. cited 2018 Dec 3. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.7. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490:61–70. PMID: 23000897.8. Lips EH, Michaut M, Hoogstraat M, Mulder L, Besselink NJ, Koudijs MJ, et al. Next generation sequencing of triple negative breast cancer to find predictors for chemotherapy response. Breast Cancer Res. 2015; 17:134. PMID: 26433948.9. Bae SY, Nam SJ, Jung Y, Lee SB, Park BW, Lim W, et al. Differences in prognosis and efficacy of chemotherapy by p53 expression in triple-negative breast cancer. Breast Cancer Res Treat. 2018; 172:437–444. PMID: 30132220.10. Bae SY, Jung SP, Lee SK, Yu J, Lee JE, Kim SW, et al. Prognostic value of immunohistochemically detected p53 in adjuvant chemotherapy-treated triple negative breast cancer. Kaohsiung J Med Sci. 2018; 34:663–672. PMID: 30527200.11. Cho DH, Bae SY, You JY, Kim HK, Chang YW, Choi YJ, et al. Lymph node ratio as an alternative to pN staging for predicting prognosis after neoadjuvant chemotherapy in breast cancer. Kaohsiung J Med Sci. 2018; 34:341–347. PMID: 29747778.12. Fountzilas G, Giannoulatou E, Alexopoulou Z, Zagouri F, Timotheadou E, Papadopoulou K, et al. TP53 mutations and protein immunopositivity may predict for poor outcome but also for trastuzumab benefit in patients with early breast cancer treated in the adjuvant setting. Oncotarget. 2016; 7:32731–32753. PMID: 27129168.13. Lara JF, Thor AD, Dressler LG, Broadwater G, Bleiweiss IJ, Edgerton S, et al. p53 Expression in node-positive breast cancer patients: results from the Cancer and Leukemia Group B 9344 Trial (159905). Clin Cancer Res. 2011; 17:5170–5178. PMID: 21693655.14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.15. Bonnefoi H, Litiere S, Piccart M, MacGrogan G, Fumoleau P, Brain E, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014; 25:1128–1136. PMID: 24618153.16. Wang Y, Xu Y, Chen J, Ouyang T, Li J, Wang T, et al. TP53 mutations are associated with higher rates of pathologic complete response to anthracycline/cyclophosphamide-based neoadjuvant chemotherapy in operable primary breast cancer. Int J Cancer. 2016; 138:489–496. PMID: 26238069.17. Darb-Esfahani S, Denkert C, Stenzinger A, Salat C, Sinn B, Schem C, et al. Role of TP53 mutations in triple negative and HER2-positive breast cancer treated with neoadjuvant anthracycline/taxane-based chemotherapy. Oncotarget. 2016; 7:67686–67698. PMID: 27611952.18. Knappskog S, Berge EO, Chrisanthar R, Geisler S, Staalesen V, Leirvaag B, et al. Concomitant inactivation of the p53- and pRB- functional pathways predicts resistance to DNA damaging drugs in breast cancer in vivo. Mol Oncol. 2015; 9:1553–1564. PMID: 26004085.19. Lee HC, Ko H, Seol H, Noh DY, Han W, Kim TY, et al. Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response. J Breast Cancer. 2013; 16:395–403. PMID: 24454461.20. Kim T, Han W, Kim MK, Lee JW, Kim J, Ahn SK, et al. Predictive significance of p53, Ki-67, and Bcl-2 expression for pathologic complete response after neoadjuvant chemotherapy for triple-negative breast cancer. J Breast Cancer. 2015; 18:16–21. PMID: 25834606.21. Jiang YZ, Yu KD, Bao J, Peng WT, Shao ZM. Favorable prognostic impact in loss of TP53 and PIK3CA mutations after neoadjuvant chemotherapy in breast cancer. Cancer Res. 2014; 74:3399–3407. PMID: 24924774.22. Varna M, Lehmann-Che J, Turpin E, Marangoni E, El-Bouchtaoui M, Jeanne M, et al. p53 dependent cell-cycle arrest triggered by chemotherapy in xenografted breast tumors. Int J Cancer. 2009; 124:991–997. PMID: 19048622.23. Jackson JG, Pant V, Li Q, Chang LL, Quintas-Cardama A, Garza D, et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell. 2012; 21:793–806. PMID: 22698404.24. Chrisanthar R, Knappskog S, Lokkevik E, Anker G, Ostenstad B, Lundgren S, et al. Predictive and prognostic impact of TP53 mutations and MDM2 promoter genotype in primary breast cancer patients treated with epirubicin or paclitaxel. PLoS One. 2011; 6:e19249. PMID: 21556366.25. Geisler S, Lonning PE, Aas T, Johnsen H, Fluge O, Haugen DF, et al. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res. 2001; 61:2505–2512. PMID: 11289122.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- The Effect of Neoadjuvant Chemotherapy on the Biological Prognostic Markers in Breast Cancer Patients
- Predictive Significance of p53, Ki-67, and Bcl-2 Expression for Pathologic Complete Response after Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Pathologic Findings of Residual Tumor according to the Response Rate after Neoadjuvant Chemotherapy for Breast Cancer