Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with synthetic cell binding peptide sequences
- Affiliations
-
- 1Department of Prosthodontics, Yonsei University College of Dentistry, Seoul, Korea.
- 2Department of Dental Biomaterials and Bioengineering, Research Institute of Yonsei University College of Dentistry, Seoul, Korea.
- 3Department of Dental Biomaterials, Wonkwang University School of Dentistry, Iksan, Korea.
- 4Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. kst72@snu.ac.kr
- KMID: 2212076
- DOI: http://doi.org/10.5051/jpis.2012.42.5.166
Abstract
- PURPOSE
The aim of this study was to evaluate the improvement of osteogenic potential of biphasic calcium phosphate (BCP) bone substitute coated with synthetic cell-binding peptide sequences in a standardized rabbit sinus model.
METHODS
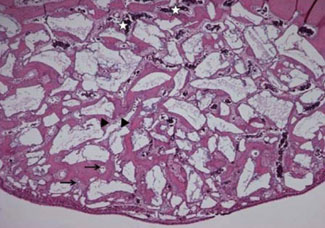
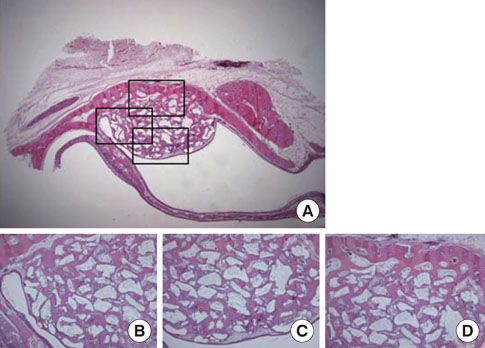
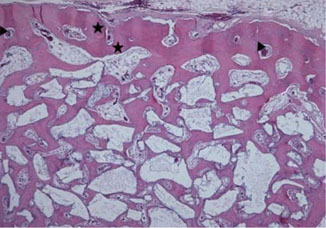
Standardized 6-mm diameter defects were created bilaterally on the maxillary sinus of ten male New Zealand white rabbits, receiving BCP bone substitute coated with synthetic cell binding peptide sequences on one side (experimental group) and BCP bone substitute without coating (control group) on the other side. Histologic and histomorphometric analysis of bone formation was carried out after a healing period of 4 or 8 weeks.
RESULTS
Histological analysis revealed signs of new bone formation in both experimental groups (4- and 8-week healing groups) with a statistically significant increase in bone formation in the 4-week healing group compared to the control group. However, no statistically significant difference in bone formation was found between the 8-week healing group and the control group.
CONCLUSIONS
This study found that BCP bone substitute coated with synthetic cell-binding peptide sequences enhanced osteoinductive potential in a standardized rabbit sinus model and its effectiveness was greater in the 4-week healing group than in the 8-week healing group.
MeSH Terms
Figure
Cited by 4 articles
-
Comparative analysis of carrier systems for delivering bone morphogenetic proteins
Im-Hee Jung, Hyun-Chang Lim, Eun-Ung Lee, Jung-Seok Lee, Ui-Won Jung, Seong-Ho Choi
J Periodontal Implant Sci. 2015;45(4):136-144. doi: 10.5051/jpis.2015.45.4.136.Four-week histologic evaluation of grafted calvarial defects with adjunctive hyperbaric oxygen therapy in rats
Hyeyoon Chang, Seo-Eun Oh, Seunghan Oh, Kyung-Seok Hu, Sungtae Kim
J Periodontal Implant Sci. 2016;46(4):244-253. doi: 10.5051/jpis.2016.46.4.244.Improvement of the osteogenic potential of ErhBMP-2-/EGCG-coated biphasic calcium phosphate bone substitute: in vitro and in vivo activity
Jae-ho Hwang, Seunghan Oh, Sungtae Kim
J Periodontal Implant Sci. 2019;49(2):114-126. doi: 10.5051/jpis.2019.49.2.114.Adjunctive hyperbaric oxygen therapy for irradiated rat calvarial defects
Heesuk An, Jung-Tae Lee, Seo-Eun Oh, Kyeong-mee Park, Kyung-Seok Hu, Sungtae Kim, Moon-Kyu Chung
J Periodontal Implant Sci. 2019;49(1):2-13. doi: 10.5051/jpis.2019.49.1.2.
Reference
-
1. Klijn RJ, Meijer GJ, Bronkhorst EM, Jansen JA. A meta-analysis of histomorphometric results and graft healing time of various biomaterials compared to autologous bone used as sinus floor augmentation material in humans. Tissue Eng Part B Rev. 2010. 16:493–507.
Article2. Klijn RJ, Meijer GJ, Bronkhorst EM, Jansen JA. Sinus floor augmentation surgery using autologous bone grafts from various donor sites: a meta-analysis of the total bone volume. Tissue Eng Part B Rev. 2010. 16:295–303.
Article3. Schlegel KA, Schultze-Mosgau S, Wiltfang J, Neukam FW, Rupprecht S, Thorwarth M. Changes of mineralization of free autogenous bone grafts used for sinus floor elevation. Clin Oral Implants Res. 2006. 17:673–678.
Article4. Schmitt CM, Doering H, Schmidt T, Lutz R, Neukam FW, Schlegel KA. Histological results after maxillary sinus augmentation with Straumann® BoneCeramic, Bio-Oss®, Puros®, and autologous bone. A randomized controlled clinical trial. Clin Oral Implants Res. 2012. 02. 13. [Epub]. http://dx.doi.org/10.1111/j.1600-0501.2012.02431.x.
Article5. Frenken JW, Bouwman WF, Bravenboer N, Zijderveld SA, Schulten EA, ten Bruggenkate CM. The use of Straumann Bone Ceramic in a maxillary sinus floor elevation procedure: a clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin Oral Implants Res. 2010. 21:201–208.
Article6. Cordaro L, Bosshardt DD, Palattella P, Rao W, Serino G, Chiapasco M. Maxillary sinus grafting with Bio-Oss or Straumann Bone Ceramic: histomorphometric results from a randomized controlled multicenter clinical trial. Clin Oral Implants Res. 2008. 19:796–803.
Article7. Kim S, Jung UW, Lee YK, Choi SH. Effects of biphasic calcium phosphate bone substitute on circumferential bone defects around dental implants in dogs. Int J Oral Maxillofac Implants. 2011. 26:265–273.8. Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003. 14:195–200.9. Kim JW, Choi KH, Yun JH, Jung UW, Kim CS, Choi SH, et al. Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011. 112:298–306.
Article10. Park JC, So SS, Jung IH, Yun JH, Choi SH, Cho KS, et al. Induction of bone formation by Escherichia coli-expressed recombinant human bone morphogenetic protein-2 using block-type macroporous biphasic calcium phosphate in orthotopic and ectopic rat models. J Periodontal Res. 2011. 46:682–690.
Article11. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008. 8:1011–1018.
Article12. Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000. 27:479–486.
Article13. Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976). 2006. 31:2813–2819.
Article14. Park JB, Lee JY, Park HN, Seol YJ, Park YJ, Rhee SH, et al. Osteopromotion with synthetic oligopeptide-coated bovine bone mineral in vivo. J Periodontol. 2007. 78:157–163.
Article15. Nam HW, Park JB, Lee JY, Rhee SH, Lee SC, Koo KT, et al. Enhanced ridge preservation by bone mineral bound with collagen-binding synthetic oligopeptide: a clinical and histologic study in humans. J Periodontol. 2011. 82:471–480.
Article16. Kim TI, Jang JH, Lee YM, Rhyu IC, Chung CP, Han SB, et al. Biomimetic approach on human periodontal ligament cells using synthetic oligopeptides. J Periodontol. 2004. 75:925–932.
Article17. Hagel-Bradway S, Dziak R. Regulation of bone cell metabolism. J Oral Pathol Med. 1989. 18:344–351.
Article18. Seyedin SM. Osteoinduction: a report on the discovery and research of unique protein growth factors mediating bone development. Oral Surg Oral Med Oral Pathol. 1989. 68(4 Pt 2):527–529.
Article19. Hole BB, Schwarz JA, Gilbert JL, Atkinson BL. A study of biologically active peptide sequences (P-15) on the surface of an ABM scaffold (PepGen P-15) using AFM and FTIR. J Biomed Mater Res A. 2005. 74:712–721.
Article20. Qian JJ, Bhatnagar RS. Enhanced cell attachment to anorganic bone mineral in the presence of a synthetic peptide related to collagen. J Biomed Mater Res. 1996. 31:545–554.
Article21. Hanks T, Atkinson BL. Comparison of cell viability on anorganic bone matrix with or without P-15 cell binding peptide. Biomaterials. 2004. 25:4831–4836.
Article22. Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, et al. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. J Periodontol. 1998. 69:655–663.
Article23. Ijiri S, Yamamuro T, Nakamura T, Kotani S, Notoya K. Effect of sterilization on bone morphogenetic protein. J Orthop Res. 1994. 12:628–636.
Article24. Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clin Oral Implants Res. 2002. 13:405–409.
Article25. Seol YJ, Park YJ, Lee SC, Kim KH, Lee JY, Kim TI, et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J Biomed Mater Res A. 2006. 77:599–607.
Article26. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004. 22:233–241.
Article27. Hwang CJ, Vaccaro AR, Lawrence JP, Hong J, Schellekens H, Alaoui-Ismaili MH, et al. Immunogenicity of bone morphogenetic proteins. J Neurosurg Spine. 2009. 10:443–451.
Article28. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009. 40:Suppl 3. S32–S38.
Article29. Saito A, Suzuki Y, Kitamura M, Ogata S, Yoshihara Y, Masuda S, et al. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J Biomed Mater Res A. 2006. 77:700–706.30. Suzuki Y, Tanihara M, Suzuki K, Saitou A, Sufan W, Nishimura Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J Biomed Mater Res. 2000. 50:405–409.
Article31. Lee CK, Koo KT, Park YJ, Lee JY, Rhee SH, Ku Y, et al. Biomimetic surface modification using synthetic oligopeptides for enhanced guided bone regeneration in beagles. J Periodontol. 2012. 83:101–110.
Article32. De Souza Nunes LS, De Oliveira RV, Holgado LA, Nary Filho H, Ribeiro DA, Matsumoto MA. Immunoexpression of Cbfa-1/Runx2 and VEGF in sinus lift procedures using bone substitutes in rabbits. Clin Oral Implants Res. 2010. 21:584–590.
Article33. Roberts EG, Breznak N. Mish CE, editor. Bone Physiology and Metabolism. Contemporary implant dentistry. 1994. Orlando: Mosby Year Book;557–598.34. Bhatnagar RS, Qian JJ, Gough CA. The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: structural and biological evidence for conformational tautomerism on fiber surface. J Biomol Struct Dyn. 1997. 14:547–560.
Article35. Valentin AH, Weber J. Receptor technology--cell binding to P-15: a new method of regenerating bone quickly and safely-preliminary histomorphometrical and mechanical results in sinus floor augmentations. Keio J Med. 2004. 53:166–171.
Article36. Bhatnagar RS, QiannJJ , Wedychowska A, Dixon E, Smith N. Biomimetic habitats for cells: ordered matrix deposition and differentiation in gingival fibroblasts cultured on hydroxyapatitie coated with a collagen analogue. Cell Mater. 1999. 9:93–104.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improvement of the osteogenic potential of ErhBMP-2-/EGCG-coated biphasic calcium phosphate bone substitute: in vitro and in vivo activity
- Comparison of the Bone Union Rates Using a Local Autobone and Bone Graft Substitute Mixed Graft in Lumbar Posterolateral Fusion
- Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with two concentrations of expressed recombinant human bone morphogenetic protein 2
- Effects of biphasic calcium phosphate on bone formation in human fetal osteoblasts
- The Effect of Recombinant Human Bone Morphogenetic Protein-2/Macroporous Biphasic Calcium Phosphate Block system on Bone Formation in Rat Calvarial Defects