Yonsei Med J.
2019 May;60(5):429-439. 10.3349/ymj.2019.60.5.429.
Biodegradable Magnesium Alloy Stents as a Treatment for Vein Graft Restenosis
- Affiliations
-
- 1Department of Vascular and Thyroid Surgery, The First Affiliated Hospital, China Medical University, Shenyang, China. sjxin@cmu.edu.cn
- 2Institute of Metal Research, Chines Academy of Sciences, Shenyang, China.
- KMID: 2443246
- DOI: http://doi.org/10.3349/ymj.2019.60.5.429
Abstract
- PURPOSE
To explore the effects of biodegradable magnesium alloy stents (BMAS) on remodeling of vein graft (VG) anastomotic restenosis.
MATERIALS AND METHODS
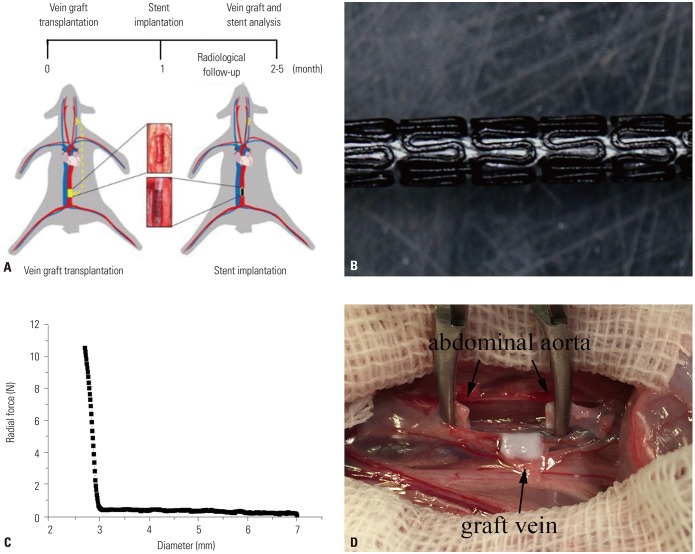
To establish a VG restenosis model, seventy two New Zealand rabbits were randomly divided into three groups according to whether a stent was implanted in the graft vein or not. BMASs and 316L stainless steel stents were implanted in BMAS and 316L groups, respectively, while no stent was implanted in the no-treatment control group (NC group). Loss of lumen diameter in the graft vein was measured in all three groups. Upon harvesting VG segments to evaluate intimal proliferation and re-endothelization, the degradation and biological safety of the stents were observed to explore the effects of BMAS on VG remodeling.
RESULTS
Model establishment and stent implantation were successful. The BMAS reduced lumen loss, compared with the control group (0.05±0.34 mm vs. 0.90±0.39 mm, p=0.001), in the early stage. The neointimal area was smaller in the BMAS group than the 316L group after 4 months (4.96±0.66 mm2 vs. 6.80±0.69 mm2, p=0.017). Re-endothelialization in the BMAS group was better than that in the 316L group (p=0.001). Within 4 months, the BMAS had degraded, and the magnesium was converted to phosphorus and calcium. The support force of the BMAS began to reduce at 2-3 months after implantation, without significant toxic effects.
CONCLUSION
BMAS promotes positive remodeling of VG anastomosis and has advantages over the conventional 316L stents in the treatment of venous diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013; 381:629–638. PMID: 23439102.
Article2. Habib RH, Dimitrova KR, Badour SA, Yammine MB, El-Hage-Sleiman AK, Hoffman DM, et al. CABG versus PCI: greater benefit in long-term outcomes with multiple arterial bypass grafting. J Am Coll Cardiol. 2015; 66:1417–1427. PMID: 26403338.3. Harskamp RE, Lopes RD, Baisden CE, de Winter RJ, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg. 2013; 257:824–833. PMID: 23574989.4. Brennan JM, Sketch MH Jr, Dai D, Trilesskaya M, Al-Hejily W, Rao SV, et al. Safety and clinical effectiveness of drug-eluting stents for saphenous vein graft intervention in older individuals: results from the medicare-linked National Cardiovascular Data Registry(®) CathPCI Registry(®) (2005–2009). Catheter Cardiovasc Interv. 2016; 87:43–49. PMID: 26153480.5. Pokala NR, Menon RV, Patel SM, Christopoulos G, Christakopoulos GE, Kotsia AP, et al. Long-term outcomes with first- vs. secondgeneration drug-eluting stents in saphenous vein graft lesions. Catheter Cardiovasc Interv. 2016; 87:34–40. PMID: 26033073.
Article6. Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010; 362:1663–1674. PMID: 20445180.
Article7. Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010; 375:201–209. PMID: 20060578.
Article8. Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart. 2003; 89:651–656. PMID: 12748224.
Article9. Haude M, Erbel R, Erne P, Verheye S, Degen H, Böse D, et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet. 2013; 381:836–844. PMID: 23332165.
Article10. Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016; 387:31–39. PMID: 26470647.
Article11. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011; 58:e44–e122. PMID: 22070834.12. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115:2344–2351. PMID: 17470709.13. Peuster M, Beerbaum P, Bach FW, Hauser H. Are resorbable implants about to become a reality? Cardiol Young. 2006; 16:107–116. PMID: 16553970.
Article14. Hermawan H, Dubé D, Mantovani D. Developments in metallic biodegradable stents. Acta Biomater. 2010; 6:1693–1697. PMID: 19815097.
Article15. Waksman R, Pakala R, Kuchulakanti PK, Baffour R, Hellinga D, Seabron R, et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter Cardiovasc Interv. 2006; 68:607–617. PMID: 16969879.
Article16. Ghimire G, Spiro J, Kharbanda R, Roughton M, Barlis P, Mason M, et al. Initial evidence for the return of coronary vasoreactivity following the absorption of bioabsorbable magnesium alloy coronary stents. EuroIntervention. 2009; 4:481–484. PMID: 19284070.
Article17. Curcio A, Torella D, Indolfi C. Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy. Circ J. 2011; 75:1287–1296. PMID: 21532177.18. Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002; 105:2974–2980. PMID: 12081990.
Article19. Sternberg K, Gratz M, Koeck K, Mostertz J, Begunk R, Loebler M, et al. Magnesium used in bioabsorbable stents controls smooth muscle cell proliferation and stimulates endothelial cells in vitro. J Biomed Mater Res B Appl Biomater. 2012; 100:41–50. PMID: 22114061.
Article20. Ma J, Zhao N, Zhu D. Biphasic responses of human vascular smooth muscle cells to magnesium ion. J Biomed Mater Res A. 2016; 104:347–356. PMID: 26402437.
Article21. Li H, Zhong H, Xu K, Yang K, Liu J, Zhang B, et al. Enhanced efficacy of sirolimus-eluting bioabsorbable magnesium alloy stents in the prevention of restenosis. J Endovasc Ther. 2011; 18:407–415. PMID: 21679083.
Article22. Maier JA, Bernardini D, Rayssiguier Y, Mazur A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim Biophys Acta. 2004; 1689:6–12. PMID: 15158908.
Article23. Zhao N, Zhu D. Endothelial responses of magnesium and other alloying elements in magnesium-based stent materials. Metallomics. 2015; 7:118–128. PMID: 25363018.
Article24. Slottow TL, Pakala R, Okabe T, Hellinga D, Lovec RJ, Tio FO, et al. Optical coherence tomography and intravascular ultrasound imaging of bioabsorbable magnesium stent degradation in porcine coronary arteries. Cardiovasc Revasc Med. 2008; 9:248–254. PMID: 18928950.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biodegradable Stent/Tube for Pancreatic and Biliary Disease
- Complete Fracture of Sirolimus-Eluting Stent in a Saphenous Vein Graft to Left Anterior Descending Artery
- Successful Management of a Rare Case of Stent Fracture and Subsequent Migration of the Fractured Stent Segment Into the Ascending Aorta in In-Stent Restenotic Lesions of a Saphenous Vein Graft
- Comparison of the Cobalt Alloy and Stainless Steel Core(r) Stent in a Porcine Coronary Restenosis Model
- Systemic drug therapy and restenosis after drug-eluting stent implantation