Dement Neurocogn Disord.
2018 Dec;17(4):163-173. 10.12779/dnd.2018.17.4.163.
¹â¸F-THK5351 PET Imaging in the Behavioral Variant of Frontotemporal Dementia
- Affiliations
-
- 1College of Medicine, Gachon University, Incheon, Korea.
- 2Neuroscience Research Institute, Gachon University, Incheon, Korea.
- 3Department of Psychiatry, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- 4Department of Neuroscience, Gachon University College of Medicine, Incheon, Korea.
- 5Department of Neurology, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea. ynoh@gachon.ac.kr, djshin@gilhospital.com
- 6Department of Health Science and Technology, Gachon Advanced Institute for Health Sciences & Technology, Gachon University, Incheon, Korea.
- KMID: 2442787
- DOI: http://doi.org/10.12779/dnd.2018.17.4.163
Abstract
- BACKGROUND AND PURPOSE
Behavioral variant frontotemporal dementia (bvFTD) is a subtype of frontotemporal dementia, which has clinical symptoms of progressive personality and behavioral changes with deterioration of social cognition and executive functions. The pathology of bvFTD is known to be tauopathy or TDP-43 equally. We analyzed the 18F-THK5351 positron emission tomography (PET) scans, which were recently developed tau PET, in patients with clinically-diagnosed bvFTD.
METHODS
Forty-eight participants, including participants with behavioral variant frontotemporal dementia (bvFTD, n=3), Alzheimer's disease (AD, n=21) and normal cognition (NC, n=24) who completed 3T magnetic resonance images, 18F-THK5351 PET scans, and detailed neuropsychological tests were included in the study. Voxel-wise statistical analysis and region of interest (ROI)-based analyses were performed to evaluate the retention of THK in bvFTD patients.
RESULTS
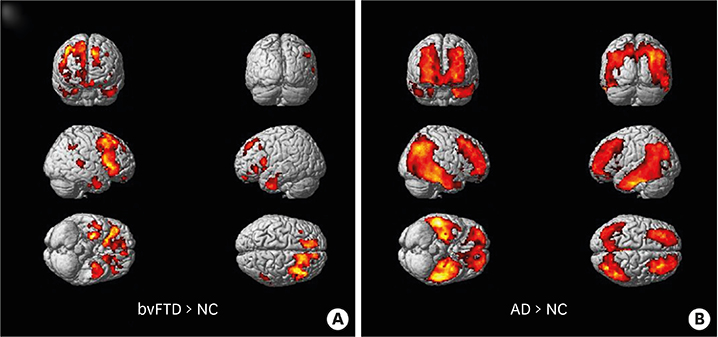
In the voxel-based and ROI-based analyses, patients with bvFTD showed greater THK retention in the prefrontal, medial frontal, orbitofrontal, anterior cingulate, insula, anterior inferior temporal and striatum regions compared to NC participants. Left-right asymmetry was noted in the bvFTD patients. A patient with extrapyramidal symptoms showed much greater THK retention in the brainstem.
CONCLUSIONS
The distribution of THK retention in the bvFTD patients was mainly in the frontal, insula, anterior temporal, and striatum regions which are known to be the brain regions corresponding to the clinical symptoms of bvFTD. Our study suggests that 18F-THK5351 PET imaging could be a supportive tool for diagnosis of bvFTD.
MeSH Terms
Figure
Reference
-
1. Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010; 119:1–4.
Article2. Mackenzie IR, Foti D, Woulfe J, Hurwitz TA. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008; 131:1282–1293.
Article3. Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005; 128:1996–2005.
Article4. Rascovsky K, Hodges J, Knopman D, Mendez M, Kramer J, Neuhaus J, et al. Can clinical features predict tau pathology in patients with behavioral variant frontotemporal dementia (bvFTD)? Neurology. 2013; 80:P05.101.5. Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. 2014; 85:865–870.
Article6. Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011; 45:384–389.
Article7. Chien DT, Szardenings AK, Bahri S, Walsh JC, Mu F, Xia C, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis. 2014; 38:171–184.
Article8. Harada R, Okamura N, Furumoto S, Furukawa K, Ishiki A, Tomita N, et al. 18F-THK5351: a novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J Nucl Med. 2016; 57:208–214.
Article9. Cho H, Seo SW, Choi JY, Lee HS, Ryu YH, Lee MS, et al. Predominant subcortical accumulation of 18F-flortaucipir binding in behavioral variant frontotemporal dementia. Neurobiol Aging. 2018; 66:112–121.
Article10. Lee MK, Hwang BY, Lee SA, Oh GJ, Choi WH, Hong SS, et al. 1-methyl-2-undecyl-4(1H)-quinolone as an irreversible and selective inhibitor of type B monoamine oxidase. Chem Pharm Bull (Tokyo). 2003; 51:409–411.
Article11. Ng KP, Pascoal TA, Mathotaarachchi S, Therriault J, Kang MS, Shin M, et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimers Res Ther. 2017; 9:25.
Article12. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011; 134:2456–2477.13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984; 34:939–944.
Article14. Srikanth S, Nagaraja AV, Ratnavalli E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer's disease, vascular dementia and frontotemporal dementia. J Neurol Sci. 2005; 236:43–48.
Article15. Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, et al. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage. 2014; 92:225–236.
Article16. Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C, et al. Different partial volume correction methods lead to different conclusions: an (18)F-FDG-PET study of aging. Neuroimage. 2016; 132:334–343.
Article17. Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016; 139:1551–1567.
Article18. Okamura N, Furumoto S, Fodero-Tavoletti MT, Mulligan RS, Harada R, Yates P, et al. Non-invasive assessment of Alzheimer's disease neurofibrillary pathology using 18F-THK5105 PET. Brain. 2014; 137:1762–1771.
Article19. Lockhart SN, Baker SL, Okamura N, Furukawa K, Ishiki A, Furumoto S, et al. Dynamic PET measures of tau accumulation in cognitively normal older adults and Alzheimer's disease patients measured using [18F] THK-5351. PLoS One. 2016; 11:e0158460.
Article20. Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014; 55:1623–1628.
Article21. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006; 7:268–277.
Article22. Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003; 23:6475–6479.
Article23. Happaney K, Zelazo PD, Stuss DT. Development of orbitofrontal function: current themes and future directions. Brain Cogn. 2004; 55:1–10.
Article24. Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994; 372:260–263.
Article25. Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2010; 22:1083–1094.
Article26. Burrell JR, Hodges JR, Rowe JB. Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov Disord. 2014; 29:684–693.
Article27. Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M. Role of astrocytes in brain function and disease. Toxicol Pathol. 2011; 39:115–123.
Article28. Broe M, Kril J, Halliday GM. Astrocytic degeneration relates to the severity of disease in frontotemporal dementia. Brain. 2004; 127:2214–2220.
Article29. Harada R, Ishiki A, Kai H, Sato N, Furukawa K, Furumoto S, et al. Correlations of 18F-THK5351 PET with postmortem burden of tau and astrogliosis in Alzheimer disease. J Nucl Med. 2018; 59:671–674.
Article30. Serrano-Pozo A, Mielke ML, Gómez-Isla T, Betensky RA, Growdon JH, Frosch MP, et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer's disease. Am J Pathol. 2011; 179:1373–1384.
Article31. Son HJ, Oh JS, Roh JH, Seo SW, Oh M, Lee SJ, et al. Differences in gray and white matter (18)F-THK5351 uptake between behavioral-variant frontotemporal dementia and other dementias. Eur J Nucl Med Mol Imaging. 2019; 46:357–366.
Article32. Jang YK, Lyoo CH, Park S, Oh SJ, Cho H, Oh M, et al. Head to head comparison of [18F] AV-1451 and [18F] THK5351 for tau imaging in Alzheimer's disease and frontotemporal dementia. Eur J Nucl Med Mol Imaging. 2018; 45:432–442.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Frontotemporal Lobe Dementia
- Severe Right Internal Carotid Artery Stenosis Mimicking Behavioral Variant Frontotemporal Dementia
- Temporal Variant of Frontotemporal Dementia: A Case of Semantic Dementia
- PET studies in Alzheimer Disease and Other Degenerative Dementias
- A Case of Frontotemporal Dementia with Family History