Korean J Ophthalmol.
2019 Apr;33(2):150-166. 10.3341/kjo.2018.0081.
Short-term Efficacy and Safety of Ranibizumab for Neovascular Age-related Macular Degeneration in the Real World: A Post-marketing Surveillance Study
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Bundang Hospital, Seongnam, Korea. sejoon1@snu.ac.kr
- 2Novartis Korea, Seoul, Korea.
- 3Hyemin Eye Hospital, Seoul, Korea.
- KMID: 2442620
- DOI: http://doi.org/10.3341/kjo.2018.0081
Abstract
- PURPOSE
To investigate the short-term efficacy and safety of ranibizumab in the routine clinical setting in patients with neovascular age-related macular degeneration and to analyze the associated factors for visual outcome.
METHODS
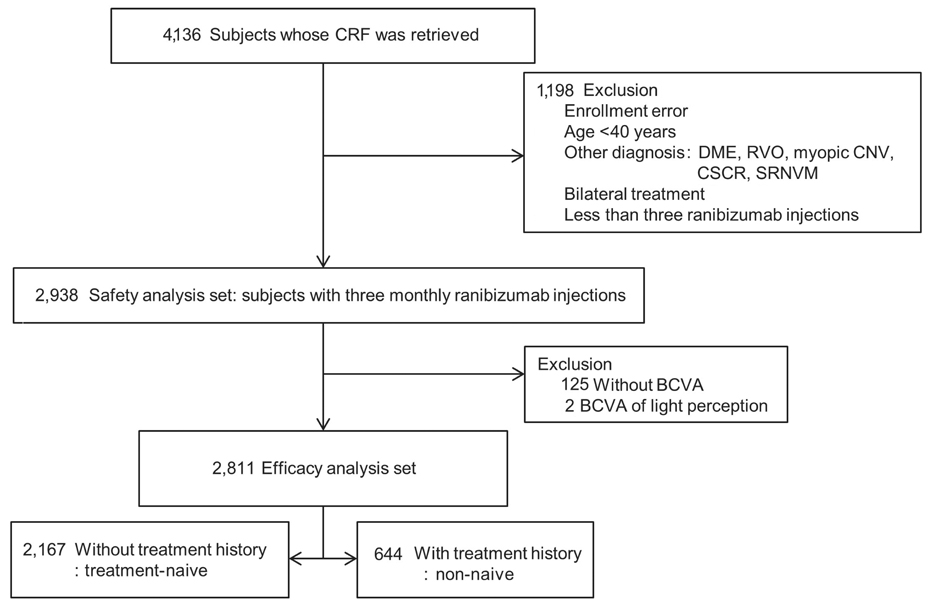
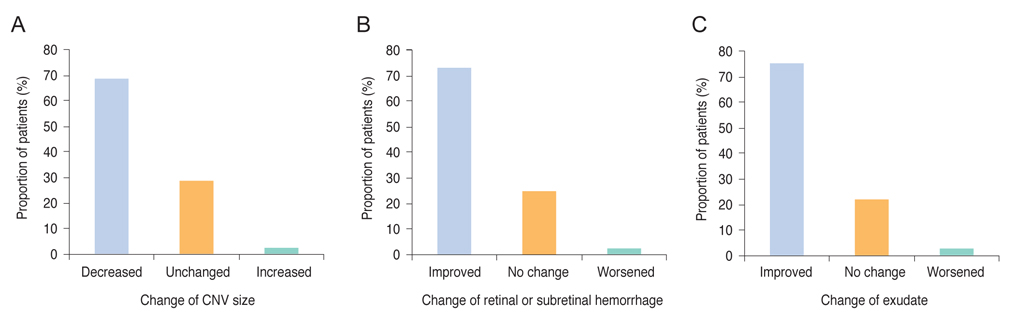
This was a post-hoc analysis of a ranibizumab regulatory post-marketing surveillance study in which 4,136 patients were enrolled and followed for 12 weeks. Change in best-corrected visual acuity (BCVA), size of choroidal neovascularization, and the presence of hemorrhage and exudate were analyzed and the association between BCVA change and baseline characteristics were investigated. Data on ocular and systemic adverse events were collected.
RESULTS
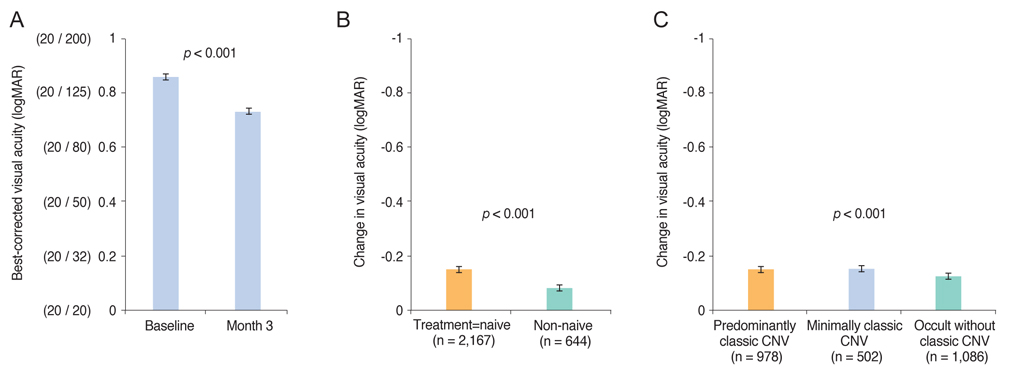
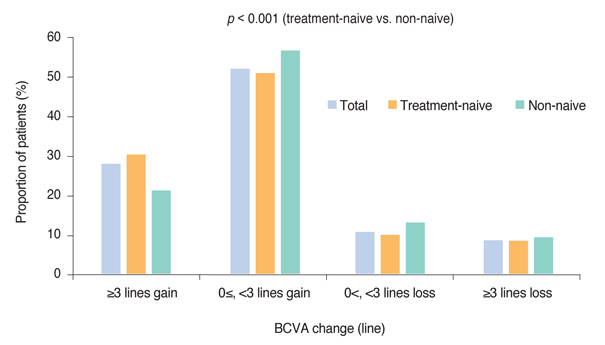
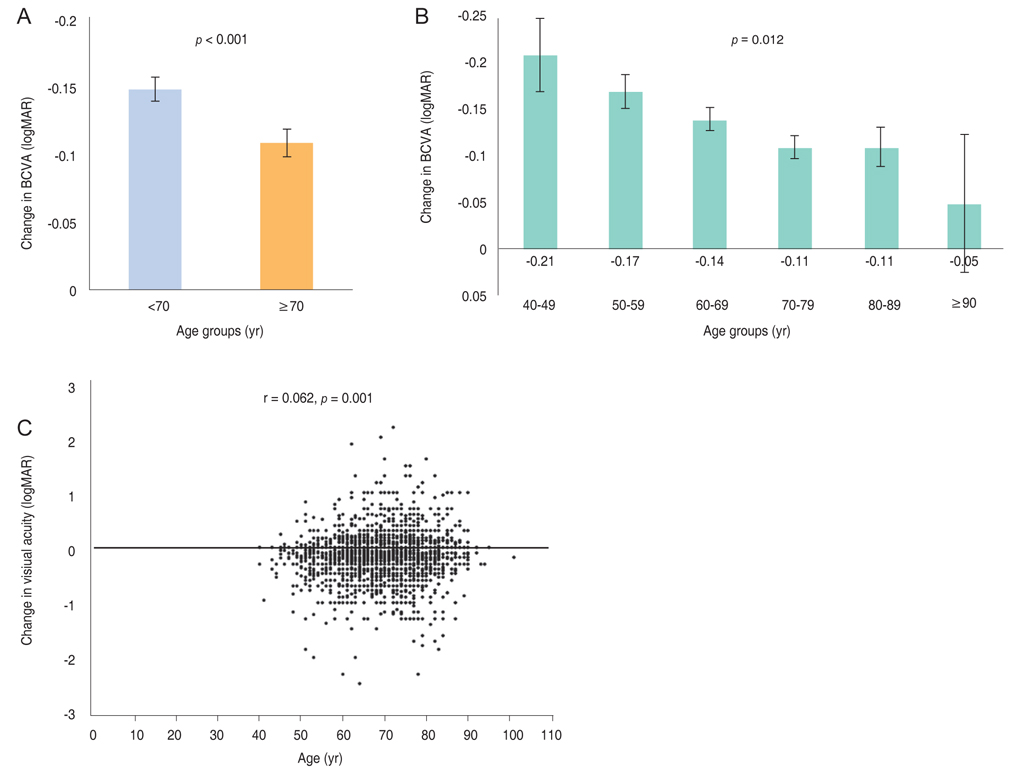
Mean BCVA improved significantly and mean BCVA change was the logarithm of the minimal angle of resolution 0.13 ± 0.01 (p < 0.001). A lower baseline BCVA and younger age were significant predictive factors for visual improvement or maintenance (≥0 lines). For greater visual acuity gain (≥3 lines), no treatment history, lower baseline BCVA, younger age, and classic-type choroidal neovascularization were significant predictive factors. No new safety signals were found.
CONCLUSIONS
In this study, conducted in real-world clinical practice with a large number of neovascular age-related macular degeneration patients, visual and anatomical outcomes improved significantly after three monthly ranibizumab treatments. Treatment-naive patients had a higher chance of greater visual gain (≥3 lines) than non-naive patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004; 291:1900–1901.2. Lee PP, Feldman ZW, Ostermann J, et al. Longitudinal prevalence of major eye diseases. Arch Ophthalmol. 2003; 121:1303–1310.
Article3. Park SJ, Lee JH, Woo SJ, et al. Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014; 121:1756–1765.4. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.
Article5. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.
Article6. Kim JH, Lee DW, Chang YS, et al. Twelve-month outcomes of treatment using ranibizumab or aflibercept for neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2016; 254:2101–2109.
Article7. Kang HM, Koh HJ. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013; 156:652–660.
Article8. Shin JY, Yu HG. Optical coherence tomography-based ranibizumab monotherapy for retinal angiomatous proliferation in Korean patients. Retina. 2014; 34:2359–2366.
Article9. Ogura Y, Terasaki H, Gomi F, et al. Efficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 study. Br J Ophthalmol. 2015; 99:92–97.
Article10. Lee FL, Kwon OW, Chung H, et al. Ranibizumab in South Korean and Taiwanese patients with age-related macular degeneration: primary outcome of the EXTEND III study. Acta Ophthalmol. 2012; 90:e406–e407.
Article11. Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997; 13:388–391.
Article12. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.
Article13. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–2548.
Article14. Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013; 120:122–129.
Article15. Ying GS, Maguire MG, Daniel E, et al. Association of baseline characteristics and early vision response with 2-year vision outcomes in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2015; 122:2523–2531.
Article16. Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014; 121:150–161.17. Grunwald JE, Pistilli M, Daniel E, et al. Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017; 124:97–104.
Article18. Grunwald JE, Pistilli M, Ying GS, et al. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015; 122:809–816.
Article19. Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007; 114:246–252.
Article20. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007; 144:850–857.
Article21. Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011; 118:523–530.
Article22. Chen M, Rajapakse D, Fraczek M, et al. Retinal pigment epithelial cell multinucleation in the aging eye: a mechanism to repair damage and maintain homoeostasis. Aging Cell. 2016; 15:436–445.23. Park SJ, Kwon KE, Choi NK, et al. Prevalence and incidence of exudative age-related macular degeneration in South Korea: a nationwide population-based study. Ophthalmology. 2015; 122:2063–2070.24. Holz FG, Bandello F, Gillies M, et al. Safety of ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. Br J Ophthalmol. 2013; 97:1161–1167.
Article25. Wolf A, Kampik A. Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol. 2014; 252:647–655.
Article26. Cohen SY, Mimoun G, Oubraham H, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013; 33:474–481.27. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–1398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Five-year Outcomes of Ranibizumab in Neovascular Age-related Macular Degeneration: Real Life Clinical Experience
- Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
- Intravitreal Ranibizumab Therapy for Neovascular Age-Related Macular Degeneration with a Predominantly Hemorrhagic Lesion
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Safety and efficacy of fimasartan with essential hypertension patients in real world clinical practice: data from a post marketing surveillance in Korea