Korean J Ophthalmol.
2017 Oct;31(5):424-430. 10.3341/kjo.2016.0125.
Five-year Outcomes of Ranibizumab in Neovascular Age-related Macular Degeneration: Real Life Clinical Experience
- Affiliations
-
- 1Beyoglu Eye Training and Research Hospital, Istanbul, Turkey. abdozkaya@gmail.com
- KMID: 2390285
- DOI: http://doi.org/10.3341/kjo.2016.0125
Abstract
- PURPOSE
To evaluate the outcomes of 5-year ranibizumab treatment in neovascular age-related macular degeneration (nAMD) in a single center and real life clinical setting.
METHODS
The records of nAMD patients who were treated with ranibizumab between January 2010 and June 2011 were retrospectively reviewed. Patients who completed 5 years of follow-up were included. Main outcome measures were change in best-corrected visual acuity, central retinal thickness, and visit and injection numbers.
RESULTS
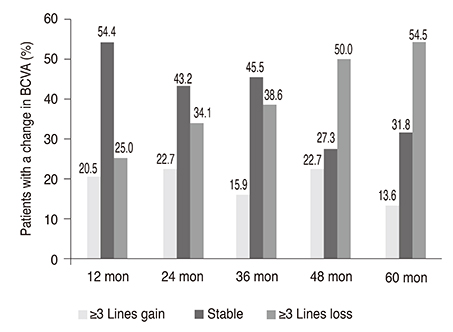
Forty-four eyes of 37 patients were included. Mean best-corrected visual acuity decreased from 0.82 ± 0.69 to 1.11 ± 0.65 logarithm of minimal angle of resolution after 5 years. Twenty-four eyes (54.5%) had visual acuity loss ≥3 lines, and 20 eyes (45.5%) had stable or improved vision (loss <3 lines, remained stable, or gained ≥1 line) at month 60. The mean total number of visits was 25.3 ± 5.8 (range, 14 to 42), and the mean total number of injections was 12.6 ± 6.4 (range, 3 to 26) at month 60.
CONCLUSIONS
Half of the ranibizumab-treated nAMD patients maintained their vision during the 5 years of follow-up. Visit and injection numbers were found to be lower than in prospective studies, reflecting a real world clinical practice.
MeSH Terms
Figure
Reference
-
1. Soubrane G, Coscas G, Francais C, Koenig F. Occult subretinal new vessels in age-related macular degeneration: natural history and early laser treatment. Ophthalmology. 1990; 97:649–657.2. Roy M, Kaiser-Kupfer M. Second eye involvement in age-related macular degeneration: a four-year prospective study. Eye (Lond). 1990; 4(Pt 6):813–818.3. Kim SH, Lee DE, Park YJ. ICG-enhanced digital angiography and photocoagulation of choroidal neovascularization in age-related macular degeneration. Korean J Ophthalmol. 1995; 9:59–65.4. Kapran Z, Ozkaya A, Uyar OM. Hemorrhagic age-related macular degeneration managed with vitrectomy, subretinal injection of tissue plasminogen activator, gas tamponade, and upright positioning. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:471–476.5. Kim HW, Kim JL, Lee MH, et al. Combined treatment of photodynamic therapy and bevacizumab for choroidal neovascularization secondary to age-related macular degeneration. Korean J Ophthalmol. 2011; 25:231–237.6. Rubin GS, Bressler NM. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Effects of verteporfin therapy on contrast on sensitivity: results from the treatment of age-related macular degeneration with photodynamic therapy (TAP) investigation-TAP report No 4. Retina. 2002; 22:536–544.7. Lee DK, Kim SH, You YS, Kwon OW. High dose intravitreal bevacizumab for refractory pigment epithelial detachment in age-related macular degeneration. Korean J Ophthalmol. 2016; 30:265–271.8. Moon DR, Lee DK, Kim SH, et al. Aflibercept treatment for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol. 2015; 29:226–232.9. Ozkaya A, Alagoz C, Garip R, et al. The role of indocyanine green angiography imaging in further differential diagnosis of patients with nAMD who are morphologically poor responders to ranibizumab in a real-life setting. Eye (Lond). 2016; 30:958–965.10. Ozkaya A, Alkin Z, Yazici AT, Demirok A. Comparison of intravitreal ranibizumab in phakic and pseudophakic neovascular age-related macular degeneration patients with good baseline visual acuity. Retina. 2014; 34:853–859.11. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.12. Liu EM, Shah G, Blinder KJ, et al. Intravitreal aflibercept for neovascular AMD: short-term clinical effects of intravitreal aflibercept injection as a predictor of long-term results. Ophthalmic Surg Lasers Imaging Retina. 2015; 46:1021–1027.13. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.14. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.15. Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013; 120:2292–2299.16. Cvetkova NP, Holldobler K, Prahs P, et al. Ranibizumab in neovascular age-related macular degeneration: a 5-year follow-up. Clin Ophthalmol. 2016; 10:1047–1051.17. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Maguire MG, Martin DF, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016; 123:1751–1761.18. Ozkaya A, Alkin Z, Ozkaya HM, et al. Is spectral-domain optical coherence tomography essential for flexible treatment regimens with ranibizumab for neovascular age-related macular degeneration? J Ophthalmol. 2013; 2013:786107.19. Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration? Ophthalmology. 2012; 119:1175–1183.20. Holz FG, Tadayoni R, Beatty S, et al. Determinants of visual acuity outcomes in eyes with neovascular AMD treated with anti-VEGF agents: an instrumental variable analysis of the AURA study. Eye (Lond). 2016; 30:1063–1071.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short-term Efficacy and Safety of Ranibizumab for Neovascular Age-related Macular Degeneration in the Real World: A Post-marketing Surveillance Study
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
- Course of Neovascular Age-related Macular Degeneration that Showed Limited Response to Both Ranibizumab and Aflibercept
- Development of Subretinal Hemorrhage during Treatment of Neovascular Age-related Macular Degeneration Using a Treat-and-extend Regimen: A Case Report