Allergy Asthma Immunol Res.
2019 May;11(3):406-421. 10.4168/aair.2019.11.3.406.
Sputum Autoantibodies Are More Relevant in Autoimmune Responses in Asthma than Are Serum Autoantibodies
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, State Key Laboratory of Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. pengtao@gzhmu.edu.cn
- 2State Key Laboratory of Respiratory Disease, Sino-French Hoffmann Institute, School of Basic Medical Science, Guangzhou Medical University, Guangzhou, China. manu1991710@126.com
- KMID: 2442051
- DOI: http://doi.org/10.4168/aair.2019.11.3.406
Abstract
- PURPOSE
The data on the differences between sputum autoantibodies (Sp-Abs) and serum autoantibodies (Se-Abs) in reflection of autoimmune responses to lungs is still lacking.
METHODS
Ten types of Abs were investigated in matched Se and Sp samples collected from recruited subjects. Correlations between Ab levels and airway inflammatory parameters and measures of pulmonary function were assessed. The network-based and inter-correlated analysis was performed to explore the patterns of Sp- and Se-Ab profiles.
RESULTS
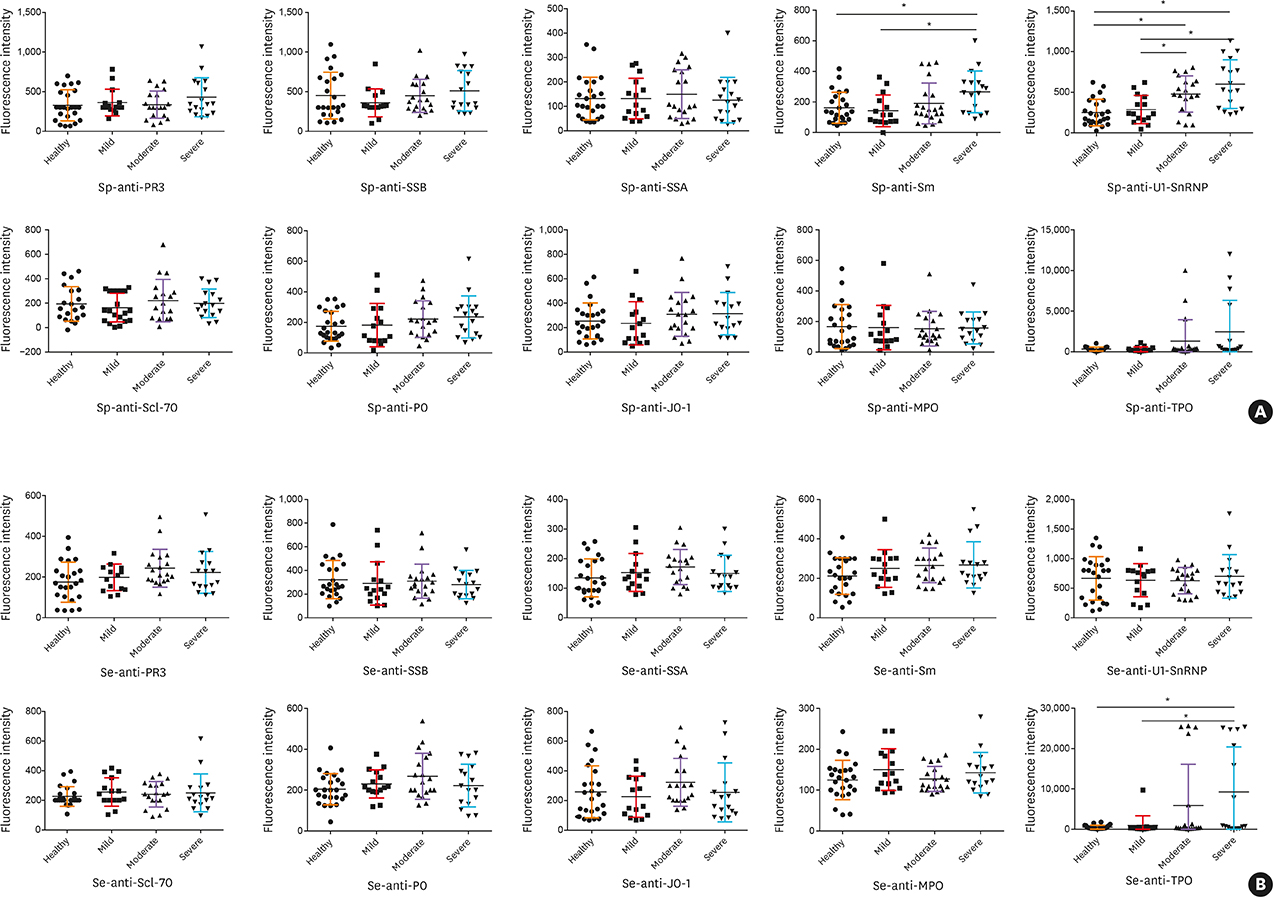
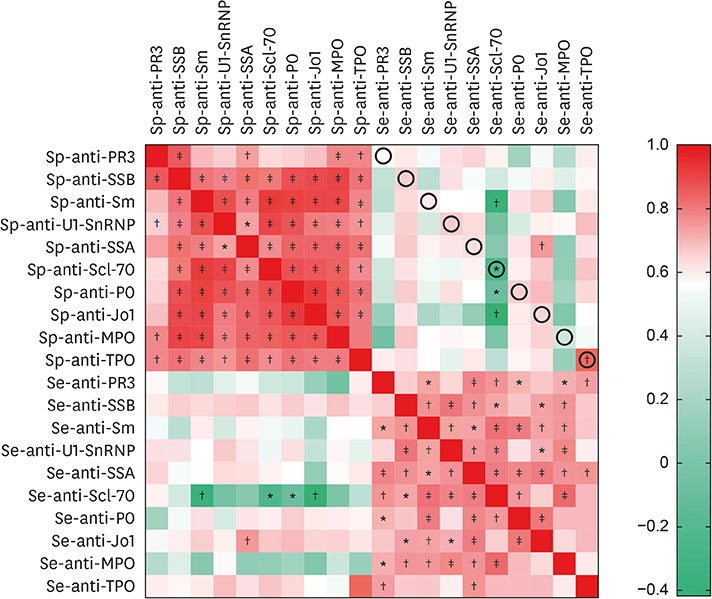
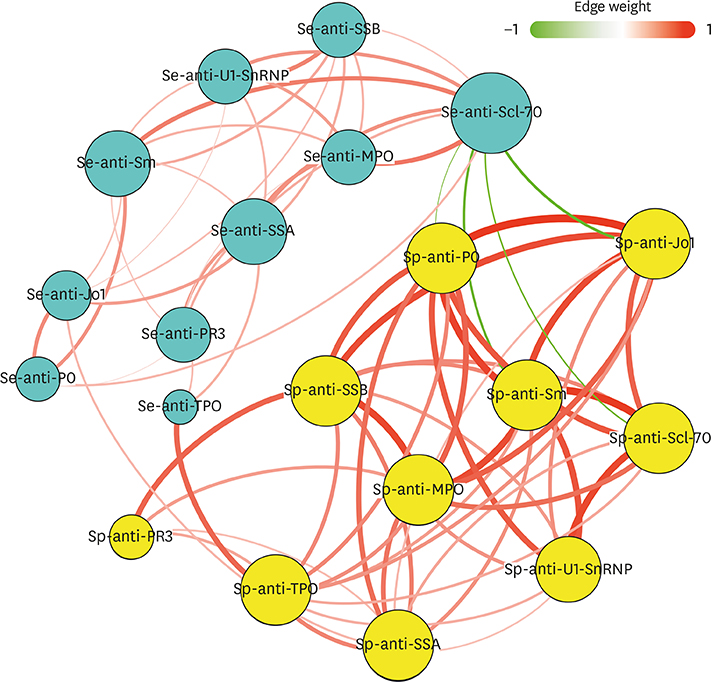
Fifty stable asthmatic patients and 24 healthy volunteers were recruited for our study, 15 with mild asthma, 18 with moderate asthma and 17 with severe asthma. The concentrations of Sp-Ab against U1 small nuclear ribonucleoprotein (Sp-anti-U1-SnRNP), Sp-Ab against Smith antigen and Se-Ab against thyroid peroxidase (anti-TPO) in severe asthmatics and Sp-anti-U1-SnRNP in moderate asthmatics were significantly higher compared to healthy controls and mild asthmatic subjects (P < 0.05). Sp-anti-U1-SnRNP levels were positively correlated with the dose of inhaled corticosteroids, Sp eosinophil counts and fractional exhaled nitric oxide (r = 0.326, P = 0.022; r = 0.356, P = 0.012; r = 0.241, P = 0.025, respectively) and negatively correlated with Sp neutrophil counts (r = −0.308, P = 0.031) with adjustment for age. Spearman's correlation matrix showed multiple inter-correlations among Sp-Abs and Se-Abs (P < 0.05) while only the levels of Ab against DNA topoisomerase and anti-TPO in Se were correlated with those Sp-Ab counterparts (P < 0.05). The network-based analysis defined 2 clusters: clusters 1 and 2 contained 10 Sp-Abs and 10 Se-Abs, respectively.
CONCLUSIONS
This study observes that Sp-Abs are more associated with clinical parameters and the severity of disease in asthma compared to Se-Abs. Targeting on Sp-Abs which are the hallmark of the localized autoimmune event might help us better understand the role of autoimmunity in the pathological mechanism of asthma.
Keyword
MeSH Terms
-
Adrenal Cortex Hormones
Asthma*
Autoantibodies*
Autoimmunity*
DNA Topoisomerases, Type I
Eosinophils
Healthy Volunteers
Humans
Iodide Peroxidase
Lung
Neutrophils
Nitric Oxide
Ribonucleoproteins, Small Nuclear
Sputum*
Adrenal Cortex Hormones
Autoantibodies
DNA Topoisomerases, Type I
Iodide Peroxidase
Nitric Oxide
Ribonucleoproteins, Small Nuclear
Figure
Reference
-
1. Stene LC, Nafstad P. Relation between occurrence of type 1 diabetes and asthma. Lancet. 2001; 357:607–608.
Article2. Tedeschi A, Airaghi L. Common risk factors in type 1 diabetes and asthma. Lancet. 2001; 357:1622.
Article3. Tedeschi A, Airaghi L. Is affluence a risk factor for bronchial asthma and type 1 diabetes? Pediatr Allergy Immunol. 2006; 17:533–537.
Article4. Sheikh A, Smeeth L, Hubbard R. There is no evidence of an inverse relationship between TH2-mediated atopy and TH1-mediated autoimmune disorders: lack of support for the hygiene hypothesis. J Allergy Clin Immunol. 2003; 111:131–135.
Article5. Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001; 108:781–783.
Article6. Bünder R, Mittermann I, Herz U, Focke M, Wegmann M, Valenta R, et al. Induction of autoallergy with an environmental allergen mimicking a self protein in a murine model of experimental allergic asthma. J Allergy Clin Immunol. 2004; 114:422–428.
Article7. Tamai K, Yoshimatsu H, Saito T, Matsuoka H, Okada N, Koma Y, et al. Autoantibody profiles and their association with blood eosinophils in asthma and COPD. Allergol Int. 2017; 66:332–337.
Article8. Wu J, Yin K, Yang Y. Detection of circulating autoantibodies to beta 2-adrenergic receptors in patients with asthma. Zhonghua Nei Ke Za Zhi. 1997; 36:238–241.9. Hall R, Turner-Warwick M, Doniach D. Autoantibodies in iodide goitre and asthma. Clin Exp Immunol. 1966; 1:285–296.10. Nahm DH, Lee YE, Yim EJ, Park HS, Yim H, Kang Y, et al. Identification of cytokeratin 18 as a bronchial epithelial autoantigen associated with nonallergic asthma. Am J Respir Crit Care Med. 2002; 165:1536–1539.
Article11. Liu M, Subramanian V, Christie C, Castro M, Mohanakumar T. Immune responses to self-antigens in asthma patients: clinical and immunopathological implications. Hum Immunol. 2012; 73:511–516.
Article12. Nahm DH, Shin MJ, Yim H, Kang Y, Choi DC, Kim JK, et al. Increased levels of circulating autoantibodies to cultured human bronchial epithelial cell in adult patients with nonatopic asthma. J Korean Med Sci. 2001; 16:407–410.
Article13. Golikova EA, Lopatnikova JA, Kovalevskaya-Kucheryavenko TV, Nepomnyashih VM, Sennikov SV. Levels of TNF, TNF autoantibodies and soluble TNF receptors in patients with bronchial asthma. J Asthma. 2013; 50:705–711.
Article14. Taillé C, Grootenboer-Mignot S, Estellat C, Roy C, Ly Ka So S, Pretolani M, et al. Perip7lakin is a target for autoimmunity in asthma. Respir Res. 2016; 17:126.15. Rottem M, Shoenfeld Y. Asthma as a paradigm for autoimmune disease. Int Arch Allergy Immunol. 2003; 132:210–214.
Article16. Tedeschi A, Asero R. Asthma and autoimmunity: a complex but intriguing relation. Expert Rev Clin Immunol. 2008; 4:767–776.
Article17. Spencer CY, Millman J, Veiga K, Vicencio AG. Airway autoimmune inflammatory response (AAIR) syndrome: an asthma-autoimmune overlap disorder? Pediatrics. 2018; 141:e20170138.
Article18. Mukherjee M, Bulir DC, Radford K, Kjarsgaard M, Huang CM, Jacobsen EA, et al. Sputum autoantibodies in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2018; 141:1269–1279.
Article19. Hu ZD, Deng AM. Autoantibodies in pre-clinical autoimmune disease. Clin Chim Acta. 2014; 437:14–18.
Article20. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention [Internet]. China: Global Initiative for Asthma;2018. cited 2018 Aug 11. Available from: http://www.ginasthma.org.21. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.22. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.23. Beeh KM, Beier J, Kornmann O, Mander A, Buhl R. Long-term repeatability of induced sputum cells and inflammatory markers in stable, moderately severe COPD. Chest. 2003; 123:778–783.24. Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of community hierarchies in large networks. J Stat Mech. 2008.25. Divo MJ, Casanova C, Marin JM, Pinto-Plata VM, de-Torres JP, Zulueta JJ, et al. COPD comorbidities network. Eur Respir J. 2015; 46:640–650.
Article26. Serafini U, Torrigiani G, Masala C. Organ-specific autoantibodies in reagin-containing sera from allergic patients. Lancet. 1965; 2:821–822.
Article27. Mukherjee M, Lim HF, Thomas S, Miller D, Kjarsgaard M, Tan B, et al. Airway autoimmune responses in severe eosinophilic asthma following low-dose Mepolizumab therapy. Allergy Asthma Clin Immunol. 2017; 13:2.
Article28. Schiøtz PO, Egeskjold EM, Høiby N, Permin H. Autoantibodies in serum and sputum from patients with cystic fibrosis. Acta Pathol Microbiol Scand C. 1979; 87:319–324.29. Lacedonia D, Palladino GP, Foschino-Barbaro MP, Scioscia G, Carpagnano GE. Expression profiling of miRNA-145 and miRNA-338 in serum and sputum of patients with COPD, asthma, and asthma-COPD overlap syndrome phenotype. Int J Chron Obstruct Pulmon Dis. 2017; 12:1811–1817.30. Manoussakis MN, Tzioufas AG, Silis MP, Pange PJ, Goudevenos J, Moutsopoulos HM. High prevalence of anti-cardiolipin and other autoantibodies in a healthy elderly population. Clin Exp Immunol. 1987; 69:557–565.31. Vadasz Z, Haj T, Kessel A, Toubi E. Age-related autoimmunity. BMC Med. 2013; 11:94.
Article32. Candore G, Di Lorenzo G, Mansueto P, Melluso M, Fradà G, Li Vecchi M, et al. Prevalence of organ-specific and non organ-specific autoantibodies in healthy centenarians. Mech Ageing Dev. 1997; 94:183–190.
Article33. Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS One. 2013; 8:e60726.
Article34. Kong YH, Kim MS, Lee DY. Comparison of the prevalence of islet autoantibodies according to age and disease duration in patients with type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab. 2013; 18:65–70.
Article35. Sánchez J, Sánchez A, Cardona R. Causal relationship between anti-TPO IgE and chronic urticaria by in vitro and in vivo tests. Allergy Asthma Immunol Res. 2019; 11:29–42.36. Samareh Fekri M, Shokoohi M, Gozashti MH, Esmailian S, Jamshidian N, Shadkam-Farokhi M, et al. Association between anti-thyroid peroxidase antibody and asthma in women. Iran J Allergy Asthma Immunol. 2012; 11:241–245.37. El Shabrawy RM, Atta AH, Rashad NM. Serum anti-TPO and TPO gene polymorphism as a predictive factor for hidden autoimmune thyroiditis in patient with bronchial asthma and allergic rhinitis. Egypt J Immunol. 2016; 23:77–86.38. Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, et al. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol. 2014; 7:1175–1185.
Article39. Weiler CR, Kita H, Hukee M, Gleich GJ. Eosinophil viability during immunoglobulin-induced degranulation. J Leukoc Biol. 1996; 60:493–501.
Article40. Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. 2018; 10:428–447.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autoimmune Responses in Severe Asthma
- Immune Cell-Mediated Autoimmune Responses in Severe Asthma
- Chronic idiopathic urticaria and antithyroid autoantibodies
- Presence of circulating autoantibodies against bronchial epithelia cell in patients with nonatopic asthma
- Autoantibodies and Polymorphism of the TNF gene in Autoimmune Diabetes Mellitus