Autoimmune Responses in Severe Asthma
- Affiliations
-
- 1Division of Respirology, Department of Medicine, St. Joseph's Healthcare Hamilton, McMaster University, Hamilton, Canada. parames@mcmaster.ca
- KMID: 2418042
- DOI: http://doi.org/10.4168/aair.2018.10.5.428
Abstract
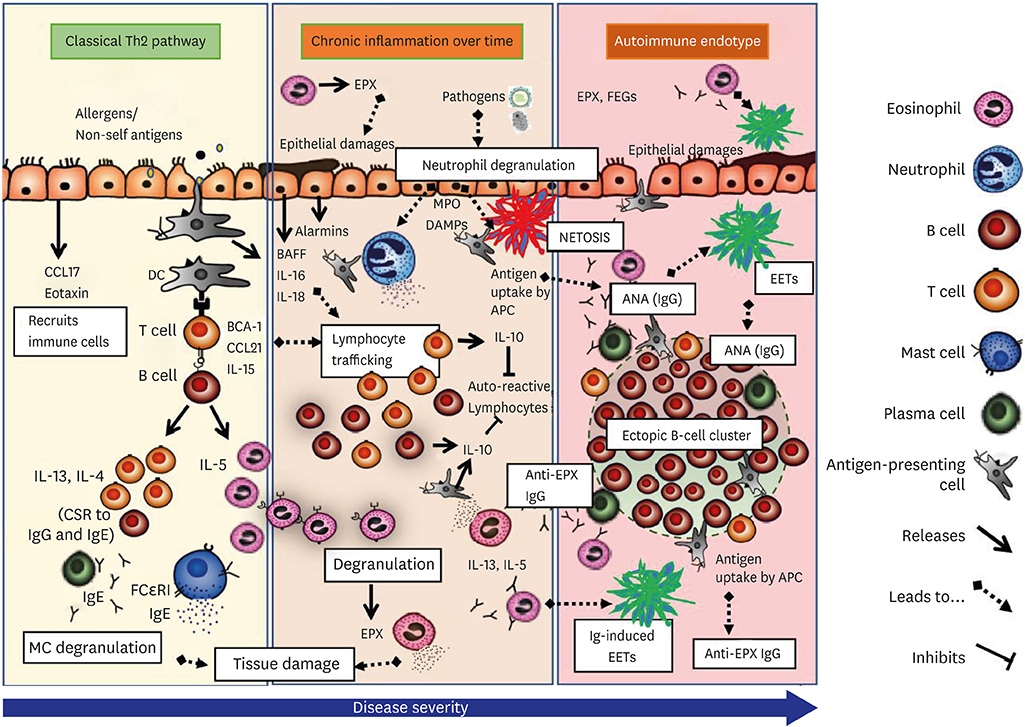
- Asthma and autoimmune diseases both result from a dysregulated immune system, and have been conventionally considered to have mutually exclusive pathogenesis. Autoimmunity is believed to be an exaggerated Th1 response, while asthma with a Th2 underpinning is congruent with the well-accepted Th1/Th2 paradigm. The hypothesis of autoimmune involvement in asthma has received much recent interest, particularly in the adult late-onset non-atopic patients (the "intrinsic asthma"). Over the past decades, circulating autoantibodies against diverse self-targets (beta-2-adrenergic receptors, epithelial antigens, nuclear antigens, etc.) have been reported and subsequently dismissed to be epiphenomena resulting from a chronic inflammatory condition, primarily due to lack of evidence of causality/pathomechanism. Recent evidence of "˜granulomas' in the lung biopsies of severe asthmatics, detection of pathogenic sputum autoantibodies against autologous eosinophil proteins (e.g., eosinophil peroxidase) and inadequate response to monoclonal antibody therapies (e.g., subcutaneous mepolizumab) in patients with evidence of airway autoantibodies suggest that the role of autoimmune mechanisms be revisited. In this review, we have gathered available reports of autoimmune responses in the lungs, reviewed the evidence in the context of immunogenic tissue-response and danger-associated molecular patterns, and constructed the possibility of an autoimmune-associated pathomechanism that may contribute to the severity of asthma.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Evaluation of Neutrophil Activation Status According to the Phenotypes of Adult Asthma
Seung-Hyun Kim, Udval Uuganbayar, Hoang Kim Tu Trinh, Duy Le Pham, Namhyo Kim, Minji Kim, Hyeukjun Sohn, Hae-Sim Park
Allergy Asthma Immunol Res. 2019;11(3):381-393. doi: 10.4168/aair.2019.11.3.381.Sputum Autoantibodies Are More Relevant in Autoimmune Responses in Asthma than Are Serum Autoantibodies
Rundong Qin, Fei Long, Xiaojun Xiao, Jing Xiao, Zhengyu Zheng, Mulin Feng, Renbin Huang, Tao Peng, Jing Li
Allergy Asthma Immunol Res. 2019;11(3):406-421. doi: 10.4168/aair.2019.11.3.406.Future Risks in Patients With Severe Asthma
Woo-Jung Song, Ji-Hyang Lee, Yewon Kang, Woo Joung Joung, Kian Fan Chung
Allergy Asthma Immunol Res. 2019;11(6):763-778. doi: 10.4168/aair.2019.11.6.763.Associated Factors for Asthma Severity in Korean Children: A Korean Childhood Asthma Study
Eun Lee, Dae Jin Song, Woo Kyung Kim, Dong In Suh, Hey-Sung Baek, Meeyong Shin, Young Yoo, Jin Tack Kim, Ji-Won Kwon, Gwang Cheon Jang, Dae Hyun Lim, Hyeon-Jong Yang, Hwan Soo Kim, Ju-Hee Seo, Sung-Il Woo, Hyung Young Kim, Youn Ho Shin, Ju Suk Lee, Jisun Yoon, Sungsu Jung, Minkyu Han, Eunjin Eom, Jinho Yu
Allergy Asthma Immunol Res. 2020;12(1):86-98. doi: 10.4168/aair.2020.12.1.86.
Reference
-
1. Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010; 160:1–9.
Article2. Rabin RL, Levinson AI. The nexus between atopic disease and autoimmunity: a review of the epidemiological and mechanistic literature. Clin Exp Immunol. 2008; 153:19–30.
Article3. Rottem M, Shoenfeld Y. Asthma as a paradigm for autoimmune disease. Int Arch Allergy Immunol. 2003; 132:210–214.
Article4. Hargreave FE, Nair P. The definition and diagnosis of asthma. Clin Exp Allergy. 2009; 39:1652–1658.
Article5. Davidson A, Diamond B. General features of autoimmune disease. In : Rose NR, Mackay IR, editors. The autoimmune diseases. 5th ed. San Diego (CA): Elsevier;2014. p. 25–36.6. Bergamaschi R, Villani S, Crabbio M, Ponzio M, Romani A, Verri A, et al. Inverse relationship between multiple sclerosis and allergic respiratory diseases. Neurol Sci. 2009; 30:115–118.
Article7. Rudwaleit M, Andermann B, Alten R, Sörensen H, Listing J, Zink A, et al. Atopic disorders in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis. 2002; 61:968–974.
Article8. Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999; 20:147–161.9. Hemminki K, Li X, Sundquist J, Sundquist K. Subsequent autoimmune or related disease in asthma patients: clustering of diseases or medical care? Ann Epidemiol. 2010; 20:217–222.
Article10. Shen TC, Tu CY, Lin CL, Wei CC, Li YF. Increased risk of asthma in patients with systemic lupus erythematosus. Am J Respir Crit Care Med. 2014; 189:496–499.
Article11. Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001; 108:781–783.
Article12. Tedeschi A, Asero R. Asthma and autoimmunity: a complex but intriguing relation. Expert Rev Clin Immunol. 2008; 4:767–776.
Article13. Venter JC, Fraser CM, Harrison LC. Autoantibodies to beta 2-adrenergic receptors: a possible cause of adrenergic hyporesponsiveness in allergic rhinitis and asthma. Science. 1980; 207:1361–1363.
Article14. Fraser CM, Venter JC, Kaliner M. Autonomic abnormalities and autoantibodies to beta-adrenergic receptors. N Engl J Med. 1981; 305:1165–1170.
Article15. Wallukat G, Wollenberger A. Autoantibodies to β2-adrenergic receptors with antiadrenergic activity from patients with allergic asthma. J Allergy Clin Immunol. 1991; 88:581–587.16. Blecher M, Lewis S, Hicks JM, Josephs S. Beta-blocking autoantibodies in pediatric bronchial asthma. J Allergy Clin Immunol. 1984; 74:246–251.
Article17. Harrison LC, Callaghan J, Venter JC, Fraser CM, Kaliner ML. Atopy, autonomic function and beta-adrenergic receptor autoantibodies. Ciba Found Symp. 1982; 248–262.18. Lassalle P, Delneste Y, Gosset P, Gras-Masse H, Wallaert B, Tonnel AB. T and B cell immune response to a 55-kDa endothelial cell-derived antigen in severe asthma. Eur J Immunol. 1993; 23:796–803.
Article19. Nahm DH, Lee YE, Yim EJ, Park HS, Yim H, Kang Y, et al. Identification of cytokeratin 18 as a bronchial epithelial autoantigen associated with nonallergic asthma. Am J Respir Crit Care Med. 2002; 165:1536–1539.
Article20. Nahm DH, Lee KH, Shin JY, Ye YM, Kang Y, Park HS. Identification of alpha-enolase as an autoantigen associated with severe asthma. J Allergy Clin Immunol. 2006; 118:376–381.21. Lee HA, Kwon B, Hur GY, Choi SJ, Nahm DH, Park HS. Isotype and IgG subclass distribution of autoantibody response to alpha-enolase protein in adult patients with severe asthma. Yonsei Med J. 2008; 49:923–930.
Article22. Kwon B, Lee HA, Choi GS, Ye YM, Nahm DH, Park HS. Increased IgG antibody-induced cytotoxicity against airway epithelial cells in patients with nonallergic asthma. J Clin Immunol. 2009; 29:517–523.
Article23. Liu M, Subramanian V, Christie C, Castro M, Mohanakumar T. Immune responses to self-antigens in asthma patients: clinical and immunopathological implications. Hum Immunol. 2012; 73:511–516.
Article24. Taillé C, Grootenboer-Mignot S, Estellat C, Roy C, Ly Ka So S, Pretolani M, et al. Periplakin is a target for autoimmunity in asthma. Respir Res. 2016; 17:126.
Article25. Taillé C, Grootenboer-Mignot S, Boursier C, Michel L, Debray MP, Fagart J, et al. Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011; 183:759–766.
Article26. Szczeklik A, Nizankowska E, Serafin A, Dyczek A, Duplaga M, Musial J. Autoimmune phenomena in bronchial asthma with special reference to aspirin intolerance. Am J Respir Crit Care Med. 1995; 152:1753–1756.
Article27. Tamai K, Yoshimatsu H, Saito T, Matsuoka H, Okada N, Koma Y, et al. Autoantibody profiles and their association with blood eosinophils in asthma and COPD. Allergol Int. 2017; 66:332–337.
Article28. Agache I, Duca L, Anghel M, Pamfil G. Antinuclear antibodies in asthma patients- a special asthma phenotype? Iran J Allergy Asthma Immunol. 2009; 49–52.29. Heinzmann A, Deichmann KA. Genes for atopy and asthma. Curr Opin Allergy Clin Immunol. 2001; 1:387–392.
Article30. Wan YI, Shrine NR, Soler Artigas M, Wain LV, Blakey JD, Moffatt MF, et al. Genome-wide association study to identify genetic determinants of severe asthma. Thorax. 2012; 67:762–768.31. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010; 363:1211–1221.
Article32. Pochini L, Scalise M, Galluccio M, Indiveri C. OCTN cation transporters in health and disease: role as drug targets and assay development. J Biomol Screen. 2013; 18:851–867.33. Kreiner E, Waage J, Standl M, Brix S, Pers TH, Couto Alves A, et al. Shared genetic variants suggest common pathways in allergy and autoimmune diseases. J Allergy Clin Immunol. 2017; 140:771–781.
Article34. Nair P, Dasgupta A, Brightling CE, Chung KF. How to diagnose and phenotype asthma. Clin Chest Med. 2012; 33:445–457.
Article35. Svenningsen S, Nair P. Asthma endotypes and an overview of targeted therapy for asthma. Front Med (Lausanne). 2017; 4:158.
Article36. Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015; 7:301ra129.37. Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009; 11:620–624.
Article38. Diny NL, Rose NR, Čiháková D. Eosinophils in autoimmune diseases. Front Immunol. 2017; 8:484.
Article39. Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994; 12:991–1045.
Article40. Matzinger P. The danger model: a renewed sense of self. Science. 2002; 296:301–305.
Article41. Ehrlich P, Morgenroth J. On haemolysins: third and fifth communications. In : Ehrlich P, Himmelweit F, Marquandt M, Dale HH, editors. The collected papers on Paul Ehrlich. London: Pergamon;1957. p. 205–255.42. Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011; 11:221–230.
Article43. Matzinger P. The evolution of the danger theory. Expert Rev Clin Immunol. 2012; 8:311–317.
Article44. Bienenstock J, Johnston N, Perey DY. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973; 28:686–692.45. Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 2010; 107:187–241.46. Salomonsson S, Larsson P, Tengnér P, Mellquist E, Hjelmström P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren's syndrome. Scand J Immunol. 2002; 55:336–342.
Article47. Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006; 116:3183–3194.
Article48. Sato A, Hayakawa H, Uchiyama H, Chida K. Cellular distribution of bronchus-associated lymphoid tissue in rheumatoid arthritis. Am J Respir Crit Care Med. 1996; 154:1903–1907.
Article49. Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013; 65:2545–2554.50. Mikuls TR, Payne JB, Deane KD, Thiele GM. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: the spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immunol. 2016; 137:28–34.
Article51. Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011; 128:1198–1206.e1.
Article52. Morissette MC, Jobse BN, Thayaparan D, Nikota JK, Shen P, Labiris NR, et al. Persistence of pulmonary tertiary lymphoid tissues and anti-nuclear antibodies following cessation of cigarette smoke exposure. Respir Res. 2014; 15:49.
Article53. Kim LH, Plaza K, Thomas SR, Draijer C, Radford K, Peters-Golden M, et al. Endogenous peroxidases in sputum interfere with horse-radish peroxidase-based ELISAs. J Immunol Methods. 2018; 454:76–79.
Article54. Wenzel SE, Vitari CA, Shende M, Strollo DC, Larkin A, Yousem SA. Asthmatic granulomatosis. Am J Respir Crit Care Med. 2012; 186:501–507.
Article55. Mukherjee M, Bulir DC, Radford K, Kjarsgaard M, Huang CM, Jacobsen EA, et al. Sputum autoantibodies in severe eosinophilic asthma. J Allergy Clin Immunol. Forthcoming 2017.56. Nair P, Ochkur SI, Protheroe C, Radford K, Efthimiadis A, Lee NA, et al. Eosinophil peroxidase in sputum represents a unique biomarker of airway eosinophilia. Allergy. 2013; 68:1177–1184.
Article57. Persson C, Uller L. Theirs but to die and do: primary lysis of eosinophils and free eosinophil granules in asthma. Am J Respir Crit Care Med. 2014; 189:628–633.
Article58. Wang J, Slungaard A. Role of eosinophil peroxidase in host defense and disease pathology. Arch Biochem Biophys. 2006; 445:256–260.
Article59. Gueirard P, Delpech A, Gilbert D, Godin M, Loet XL, Tron F. Anti-myeloperoxidase antibodies: immunological characteristics and clinical associations. J Autoimmun. 1991; 4:517–527.
Article60. McLachlan SM, Rapoport B. Thyroid peroxidase as an autoantigen. Thyroid. 2007; 17:939–948.
Article61. Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006; 6:205.
Article62. Baay-Guzman GJ, Huerta-Yepez S, Vega MI, Aguilar-Leon D, Campillos M, Blake J, et al. Role of cxcl13 in asthma: novel therapeutic target. Chest. 2012; 141:886–894.63. Kang JS, Yoon YD, Ahn JH, Kim SC, Kim KH, Kim HM, et al. B Cell-activating factor is a novel diagnosis parameter for asthma. Int Arch Allergy Immunol. 2006; 141:181–188.
Article64. Jee HM, Choi BS, Kim KW, Sohn MH, Han MY, Kim KE. Increased B cell-activating factor (BAFF) level in the sputum of children with asthma. Korean J Pediatr. 2010; 53:795–800.
Article65. Kita H, Abu-Ghazaleh R, Sanderson CJ, Gleich GJ. Effect of steroids on immunoglobulin-induced eosinophil degranulation. J Allergy Clin Immunol. 1991; 87:70–77.
Article66. Lacy P, Moqbel R. Immune effector functions of eosinophils in allergic airway inflammation. Curr Opin Allergy Clin Immunol. 2001; 1:79–84.
Article67. Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013; 121:2074–2083.
Article68. Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, et al. Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016; 137:258–267.
Article69. D'silva L, Hassan N, Wang HY, Kjarsgaard M, Efthimiadis A, Hargreave FE, et al. Heterogeneity of bronchitis in airway diseases in tertiary care clinical practice. Can Respir J. 2011; 18:144–148.70. Chu DK, Al-Garawi A, Llop-Guevara A, Pillai RA, Radford K, Shen P, et al. Therapeutic potential of anti-IL-6 therapies for granulocytic airway inflammation in asthma. Allergy Asthma Clin Immunol. 2015; 11:14.
Article71. Panda R, Krieger T, Hopf L, Renné T, Haag F, Röber N, et al. Neutrophil extracellular traps contain selected antigens of anti-neutrophil cytoplasmic antibodies. Front Immunol. 2017; 8:439.
Article72. Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy. 2013; 68:261–273.
Article73. Choi Y, Pham LD, Lee DH, Ban GY, Lee JH, Kim SH, et al. Neutrophil extracellular DNA traps induce autoantigen production by airway epithelial cells. Mediators Inflamm. 2017; 2017:5675029.
Article74. Pham DL, Ban GY, Kim SH, Shin YS, Ye YM, Chwae YJ, et al. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma. Clin Exp Allergy. 2017; 47:57–70.
Article75. Busse WW. A role for neutrophils in asthma exacerbations. Nat Med. 2017; 23:658.
Article76. Toussaint M, Jackson DJ, Swieboda D, Guedán A, Tsourouktsoglou TD, Ching YM, et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat Med. 2017; 23:681.
Article77. Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003; 81:331–371.
Article78. Mauri C, Blair PA. The incognito journey of a regulatory B cell. Immunity. 2014; 41:878–880.
Article79. Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010; 6:636–643.
Article80. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002; 3:944–950.
Article81. Donma M, Karasu E, Ozdilek B, Turgut B, Topcu B, Nalbantoglu B, et al. CD4+, CD25+, FOXP3+ T regulatory cell levels in obese, asthmatic, asthmatic obese, and healthy children. Inflammation. 2015; 38:1473–1478.82. Eusebio M, Kuna P, Kraszula L, Kupczyk M, Pietruczuk M. The relative values of CD8+CD25+Foxp3brigh Treg cells correlate with selected lung function parameters in asthma. Int J Immunopathol Pharmacol. 2015; 28:218–226.83. Takanashi S, Hasegawa Y, Kanehira Y, Yamamoto K, Fujimoto K, Satoh K, et al. Interleukin-10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J. 1999; 14:309–314.
Article84. Kawayama T, Matsunaga K, Kaku Y, Yamaguchi K, Kinoshita T, O'Byrne PM, et al. Decreased CTLA4+ and Foxp3+ CD25highCD4+ cells in induced sputum from patients with mild atopic asthma. Allergol Int. 2013; 62:203–213.85. Merayo-Chalico J, Rajme-Lopez S, Barrera-Vargas A, Alcocer-Varela J, Diaz-Zamudio M, Gomez-Martin D. Lymphopenia and autoimmunity: a double-edged sword. Hum Immunol. 2016; 77:921–929.
Article86. Barrett SP, Toh BH, Alderuccio F, van Driel IR, Gleeson PA. Organ-specific autoimmunity induced by adult thymectomy and cyclophosphamide-induced lymphopenia. Eur J Immunol. 1995; 25:238–244.
Article87. Gleeson PA, Toh BH, van Driel IR. Organ-specific autoimmunity induced by lymphopenia. Immunol Rev. 1996; 149:97–125.
Article88. Schulze-Koops H. Lymphopenia and autoimmune diseases. Arthritis Res Ther. 2004; 6:178–180.89. Mukherjee M, Svenningsen S, Nair P. Glucocortiosteroid subsensitivity and asthma severity. Curr Opin Pulm Med. 2017; 23:78–88.
Article90. Kim JT, Schimming AW, Kita H. Ligation of FcγRII (CD32) pivotally regulates survival of human eosinophils. J Immunol. 1999; 162:4253–4259.91. Druilhe A, Letuve S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003; 8:481–495.92. Chan YC, Ramadani F, Santos AF, Pillai P, Ohm-Laursen L, Harper CE, et al. “Auto-anti-IgE”: naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J Allergy Clin Immunol. 2014; 134:1394–1401.e4.
Article93. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014; CD003559.
Article94. Mukherjee M, Lim HF, Thomas S, Miller D, Kjarsgaard M, Tan B, et al. Airway autoimmune responses in severe eosinophilic asthma following low-dose Mepolizumab therapy. Allergy Asthma Clin Immunol. 2017; 13:2.
Article95. Mukherjee M, Aleman Paramo F, Kjarsgaard M, Salter B, Nair G, LaVigne N, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018; 197:38–46.
Article96. Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017; 376:2448–2458.
Article97. Sehmi R, Lim HF, Mukherjee M, Huang C, Radford K, Newbold P, et al. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J Allergy Clin Immunol. Forthcoming 2018.
Article98. Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988; 332:738–740.
Article99. van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986; 64:415–422.100. Pouliquen IJ, Howarth P, Austin D, Gunn G, Meyer E, Price RG, et al. Response to case report: Airway autoimmune responses in severe eosinophilic asthma following low-dose Mepolizumab therapy. Allergy Asthma Clin Immunol. 2017; 13:45.
Article101. Stokol T, O'Donnell P, Xiao L, Knight S, Stavrakis G, Botto M, et al. C1q governs deposition of circulating immune complexes and leukocyte Fcγ receptors mediate subsequent neutrophil recruitment. J Exp Med. 2004; 200:835–846.
Article102. Durand V, Pers JO, Renaudineau Y, Saraux A, Youinou P, Jamin C. Differential effects of anti-FcγRIIIb autoantibodies on polymorphonuclear neutrophil apoptosis and function. J Leukoc Biol. 2001; 69:233–240.103. Wermeling F, Chen Y, Pikkarainen T, Scheynius A, Winqvist O, Izui S, et al. Class A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupus. J Exp Med. 2007; 204:2259–2265.
Article104. Page R, Friday G, Stillwagon P, Skoner D, Caliguiri L, Fireman P. Asthma and selective immunoglobulin subclass deficiency: improvement of asthma after immunoglobulin replacement therapy. J Pediatr. 1988; 112:127–131.
Article105. Smiley JD, Talbert MG. High-dose intravenous gamma globulin therapy: how does it work? Am J Med Sci. 1995; 309:295–303.
Article106. Mazer BD, Gelfand EW. An open-label study of high-dose intravenous immunoglobulin in severe childhood asthma. J Allergy Clin Immunol. 1991; 87:976–983.
Article107. Gelfand EW, Landwehr LP, Esterl B, Mazer B. Intravenous immune globulin: an alternative therapy in steroid-dependent allergic diseases. Clin Exp Immunol. 1996; 104:Suppl 1. 61–66.
Article108. Kishiyama JL, Valacer D, Cunningham-Rundles C, Sperber K, Richmond GW, Abramson S, et al. A multicenter, randomized, double-blind, placebo-controlled trial of high-dose intravenous immunoglobulin for oral corticosteroid-dependent asthma. Clin Immunol. 1999; 91:126–133.
Article109. Niggemann B, Leupold W, Schuster A, Schuster R, v Berg A, Grübl A, et al. Prospective, double-blind, placebo-controlled, multicentre study on the effect of high-dose, intravenous immunoglobulin in children and adolescents with severe bronchial asthma. Clin Exp Allergy. 1998; 28:205–210.
Article110. Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Pulmonary manifestations of systemic autoimmune diseases. Maedica (Buchar). 2011; 6:224–229.111. Sinico RA, Di Toma L, Maggiore U, Bottero P, Radice A, Tosoni C, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005; 52:2926–2935.
Article112. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990; 33:1094–1100.
Article113. Pagnoux C, Guillevin L. Churg-Strauss syndrome: evidence for disease subtypes? Curr Opin Rheumatol. 2010; 22:21–28.
Article114. Cottin V, Bel E, Bottero P, Dalhoff K, Humbert M, Lazor R, et al. Respiratory manifestations of eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Eur Respir J. 2016; 48:1429–1441.
Article115. Mukherjee M, Thomas SR, Davychenko S, Lim HF, Kjarsgaard M, Radford K, et al. Sputum anti-neutrophil cytoplasmic antibodies in eosinophilic granulomatosis and polyangiitis patients with respiratory involvement. Allergy. 2017; 72:756.116. Nahm DH, Shin MJ, Yim H, Kang Y, Choi DC, Kim JK, et al. Increased levels of circulating autoantibodies to cultured human bronchial epithelial cell in adult patients with nonatopic asthma. J Korean Med Sci. 2001; 16:407–410.
Article117. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009; 360:985–993.
Article