Korean J Pediatr Infect Dis.
2001 Nov;8(2):160-167. 10.14776/kjpid.2001.8.2.160.
Immunogenicity and Safety of a Two Doses of Hepatitis A Vaccine(VAQTAâ„¢) in Healthy Children and Adolescents
- Affiliations
-
- 1Department of Pediatrics, Yongdong Severance Hospital, College of Medicine, Yonsei University, Seoul, Korea.
- KMID: 2440181
- DOI: http://doi.org/10.14776/kjpid.2001.8.2.160
Abstract
- PURPOSE
To assess the immunogenicity, safety, and tolerability of hepatitis A vaccine (VAQTAâ„¢) in healthy children and adolescents.
METHODS
Eligible subjects aged 2 to 17 years received 25 U/0.5 mL of VAQTAâ„¢ intramuscularly at 0 and 24 week schedule. Bleeds were obtained prior to vaccination and 4 weeks after the second dose to ascertain serostatus. To detect antibody to HAV after vaccination with an inactivated HA vaccine, a modification of the Abbott® HAVAB kit was used. Sample with titers ≥ 10 mIU/mL were considered seroconverted. Adverse experiences were monitored.
RESULTS
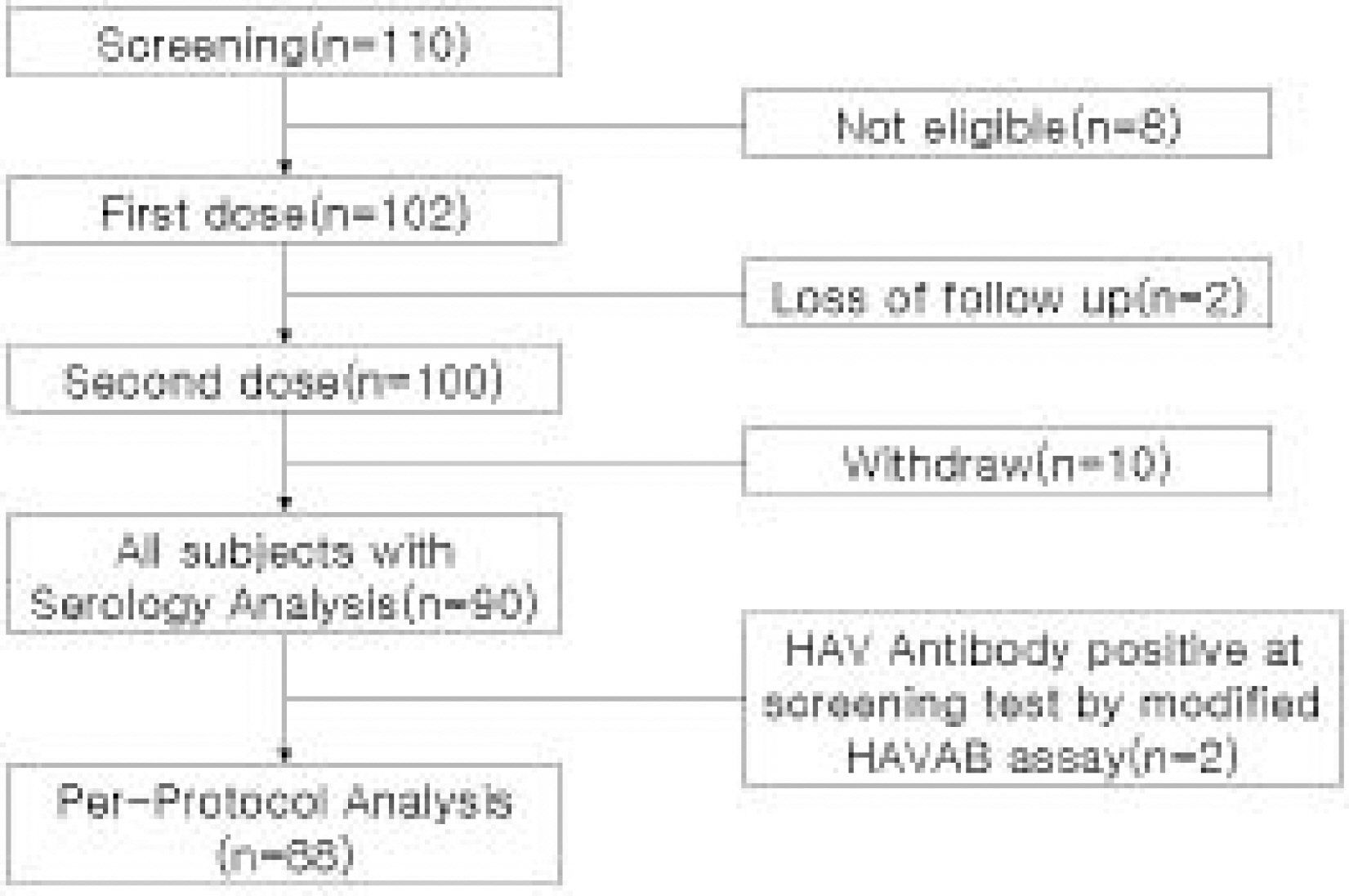
102 subjects(54 male, 48 female) were enrolled. The mean age was 6.8±3.5 years. Two subjects were seropositive, two were lost of follow up. 88 subjects were available for a per protocol analysis and 90 for all subjects with serology after the second dose, and ten withdral. All subjects(95% CI, 94.8~100) seroconverted. Geometric mean titers was 7,991.1(95% CI, 6,481.1~9,852.7) with very little difference in per protocol analysis and all subjects analysis. Adverse experiences to VAQTAâ„¢ were generally mild and transient.
CONCLUSION
The pediatric two-dose regimen of VAQTAâ„¢ was found to be highly immunogenic, generally well tolerated and resulted in 100% seroconversion. Regarding Korea is in transition from a high to low risk region resulting in a paradox increase of clinical disease and disease burden, routine vaccination should be considered in order to control hepatitis A effectively.
Keyword
MeSH Terms
Figure
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity and Safety of Recombinant Hepatitis B Vaccine(HG-II®) in Healthy Infants and Children
- Immunogenicity and Reactogenicity of Inactivated HM175 Strain Hepatitis A Vaccine in Healthy Korean Children
- Epidemiology and prevention of hepatitis B virus infection
- Reappraisal of the Immunogenicity and Safety of Three Hepatitis A Vaccines in Adolescents
- Immunogenicity and Safety of Recombinant Hepatitis B Vaccine(Engerix B)