J Korean Med Sci.
2016 Jan;31(1):73-79. 10.3346/jkms.2016.31.1.73.
Reappraisal of the Immunogenicity and Safety of Three Hepatitis A Vaccines in Adolescents
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Ewha Womans University, Seoul, Korea. kaykim@ewha.ac.kr
- 2Center for Vaccine Evaluation and Study, Medical Research Institute, School of Medicine, Ewha Womans University, Seoul, Korea.
- 3Seegene Medical Foundation, Seoul, Korea.
- 4Department of Pediatrics, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
- 5Ewha Womans University Mokdong Hospital, Seoul, Korea.
- KMID: 2359995
- DOI: http://doi.org/10.3346/jkms.2016.31.1.73
Abstract
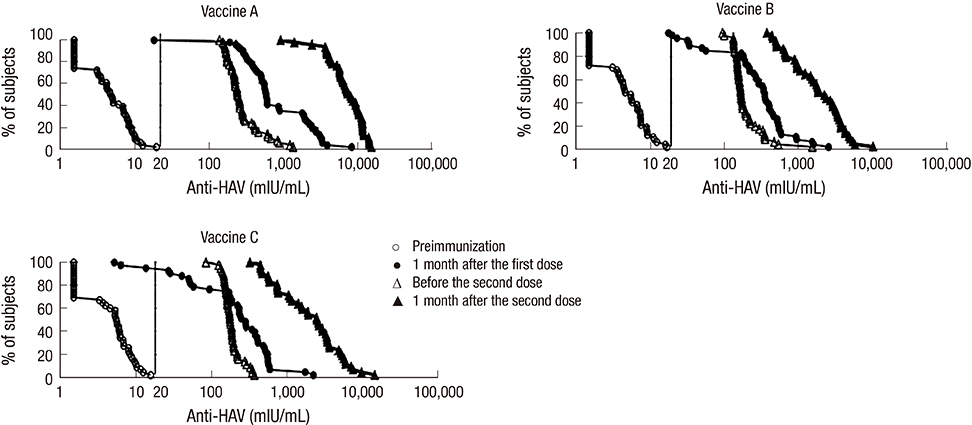
- Although the overall incidence of hepatitis A in Korea has been decreasing, adolescents remain highly vulnerable to its outbreaks. This study was conducted to compare the immunogenicity and safety of three hepatitis A vaccines in Korean adolescents. Healthy anti-hepatitis A virus seronegative subjects aged 13 to 19 yr were randomized in three equal groups to receive two doses of Avaxim(TM), Epaxal(R), or Havrix(R), 6 to 12 months apart. Seroconversion rates one month after the first dose were 98%, 95%, and 93% for Avaxim(TM), Epaxal(R), and Havrix(R), respectively. Seroconversion rates reached 100% for all vaccine groups one month after the second dose. Anti-HAV geometric mean concentrations (GMCs) were 7,207.7 mIU/mL (95% CI, 6023.1-8684.7), 1,750.5 mIU/mL (95% CI, 1362.9-2248.3), and 1,953.5 mIU/mL (95% CI, 1459.4-2614.7) after two doses of Avaxim(TM), Epaxal(R), and Havrix(R) respectively. Avaxim(TM) was significantly more immunogenic than Epaxal(R) and Havrix(R), whereas there were no significant differences in antibody responses between Epaxal(R) and Havrix(R). Local and systemic solicited adverse events (AEs) were mostly of mild-to-moderate intensity and resolved within 5 days. No serious AEs were reported. In conclusion, all three vaccines are highly immunogenic and well-tolerated in Korean adolescents. (Clinical Trial Registry NCT00483470)
Keyword
MeSH Terms
-
Adolescent
Antibody Formation
Female
Hepatitis A/immunology/*prevention & control
Hepatitis A Antibodies/blood
Hepatitis A Vaccines/adverse effects/*immunology
Humans
Male
Republic of Korea
Vaccines, Inactivated/adverse effects/immunology
Young Adult
Hepatitis A Antibodies
Hepatitis A Vaccines
Vaccines, Inactivated
Figure
Reference
-
1. Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012; 4:74.2. WHO. WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012; 87:261–276.3. Kim JH. Recent epidemiological status and vaccination of hepatitis A in Korea. J Korean Med Assoc. 2008; 51:110–118.4. Li RC, Li Y, Yi N, Huang L, Wan Z, Zhang Y, Rasuli A. An open, prospective, randomized study comparing the immunogenicity and safety of two inactivated hepatitis A pediatric vaccines in toddlers, children and adolescents in China. Pediatr Infect Dis J. 2013; 32:e77–e81.5. American Academy of Pediatrics Committee on Infectious Diseases. Hepatitis A vaccine recommendations. Pediatrics. 2007; 120:189–199.6. Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010; 28:6653–6657.7. Lee H, Cho HK, Kim JH, Kim KH. Seroepidemiology of hepatitis A in Korea: changes over the past 30 years. J Korean Med Sci. 2011; 26:791–796.8. Kim YJ, Lee HS. Increasing incidence of hepatitis A in Korean adults. Intervirology. 2010; 53:10–14.9. Jeong SH. Current status and vaccine indication for hepatitis A virus infection in Korea. Korean J Gastroenterol. 2008; 51:331–337.10. Key H. World's first hepatitis A vaccine. Inpharma Wkly. 1992; 823:14–15.11. Murphy TV, Feinstone SM. Hepatitis A vaccines. In : Plotkin SA, editor. Vaccines. Philadelphia, PA: Saunders Elsevier;2013. p. 183–204.12. Fiore AE, Wasley A, Bell BP. Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006; 55(RR-7):1–23.13. Fangcheng Z, Xuanyi W, Mingding C, Liming J, Jie W, Qi J, Yuanping G, Wen Q, Yajuan X, Jiangsen M. Era of vaccination heralds a decline in incidence of hepatitis A in high-risk groups in China. Hepat Mon. 2012; 12:100–105.14. Vidor E, Dumas R, Porteret V, Bailleux F, Veitch K. Aventis Pasteur vaccines containing inactivated hepatitis A virus: a compilation of immunogenicity data. Eur J Clin Microbiol Infect Dis. 2004; 23:300–309.15. Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines. 2008; 7:1141–1150.16. Abarca K, Ibáñez I, de la Fuente P, Cerda L, Bergeret J, Frösner G, Ibarra H. Immunogenicity and tolerability of a paediatric presentation of a virosomal hepatitis A vaccine in Chilean children aged 1-16 years. Vaccine. 2011; 29:8855–8862.17. Bovier PA, Bock J, Ebengo TF, Frösner G, Glaus J, Herzog C, Loutan L. Predicted 30-year protection after vaccination with an aluminum-free virosomal hepatitis A vaccine. J Med Virol. 2010; 82:1629–1634.18. Abarca K, Ibánez I, Perret C, Vial P, Zinsou JA. Immunogenicity, safety, and interchangeability of two inactivated hepatitis A vaccines in Chilean children. Int J Infect Dis. 2008; 12:270–277.19. Soysal A, Gokçe I, Pehlivan T, Bakir M. Interchangeability of a hepatitis A vaccine second dose: Avaxim 80 following a first dose of Vaqta 25 or Havrix 720 in children in Turkey. Eur J Pediatr. 2007; 166:533–539.20. López EL, Contrini MM, Xifró MC, Cattaneo MA, Zambrano B, Dumas R, Rouyrre N, Weber F. Hepatitis A vaccination of Argentinean infants: comparison of two vaccination schedules. Vaccine. 2007; 25:102–108.21. Lim J, Song YJ, Park WS, Sohn H, Lee MS, Shin DH, Kim CB, Kim H, Oh GJ, Ki M. The immunogenicity of a single dose of hepatitis A virus vaccines (Havrix® and Epaxal®) in Korean young adults. Yonsei Med J. 2014; 55:126–131.22. Zuckerman JN, Kirkpatrick CT, Huang M. Immunogenicity and reactogenicity of Avaxim (160 AU) as compared with Havrix (1440 EL.U) as a booster following primary immunization with Havrix (1440 EL.U) against hepatitis A. J Travel Med. 1998; 5:18–22.23. Orr N, Klement E, Gillis D, Sela T, Kayouf R, Derazne E, Grotto I, Balicer R, Huerta M, Aviram L, et al. Long-term immunity in young adults after a single dose of inactivated Hepatitis A vaccines. Vaccine. 2006; 24:4328–4332.24. Van Der Wielen M, Vertruyen A, Froesner G, Ibáñez R, Hunt M, Herzog C, Van Damme P. Immunogenicity and safety of a pediatric dose of a virosome-adjuvanted hepatitis A vaccine: a controlled trial in children aged 1-16 years. Pediatr Infect Dis J. 2007; 26:705–710.25. Dagan R, Amir J, Livni G, Greenberg D, Abu-Abed J, Guy L, Ashkenazi S, Foresner G, Tewald F, Schätzl HM, et al. Concomitant administration of a virosome-adjuvanted hepatitis A vaccine with routine childhood vaccines at age twelve to fifteen months: a randomized controlled trial. Pediatr Infect Dis J. 2007; 26:787–793.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity and Safety of a Two Doses of Hepatitis A Vaccine(VAQTAâ„¢) in Healthy Children and Adolescents
- Sleep and vaccine administration time as factors influencing vaccine immunogenicity
- Immunogenicity of a Recombinant Hepatitis B Virus Vaccine Compared with a Plasma-derived Hepatitis B Vaccine and of Vaccination Schedules in Neonates
- Immunogenicity and reactogenicity of a yeast recombinant DNA hepatitis B vaccine in healthy subjects
- Protectivity and safety following recombinant hepatitis B vaccine with different source of bulk compared to hepatitis B (Bio Farma) vaccine in Indonesia