Ann Dermatol.
2019 Apr;31(2):154-163. 10.5021/ad.2019.31.2.154.
Neuropeptides Profile and Increased Innervation in Becker's Nevus
- Affiliations
-
- 1Department of Dermatology, Dankook University College of Medicine, Cheonan, Korea. ivymyung@hanmail.net
- KMID: 2439061
- DOI: http://doi.org/10.5021/ad.2019.31.2.154
Abstract
- BACKGROUND
Melanocytes are derived from neural crest, and various pigmentary disorders may accompany abnormalities in nerve system or develop following dermatome, suggesting that melanocyte and pigmentation may be closely related to neural factors. There are reports of Becker's nevus (BN) showing linear and segmental configuration, suggesting the association of BN with nerve system. However, there are no studies regarding the expression of neuropeptides in BN.
OBJECTIVE
We investigated the expression of neuropeptides and innervation in BN.
METHODS
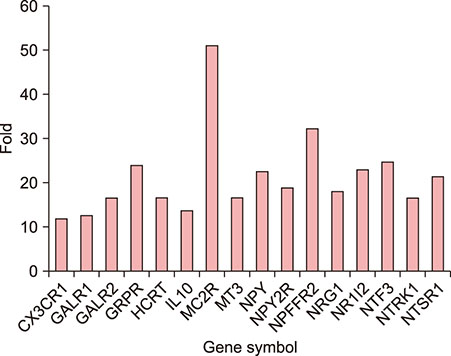
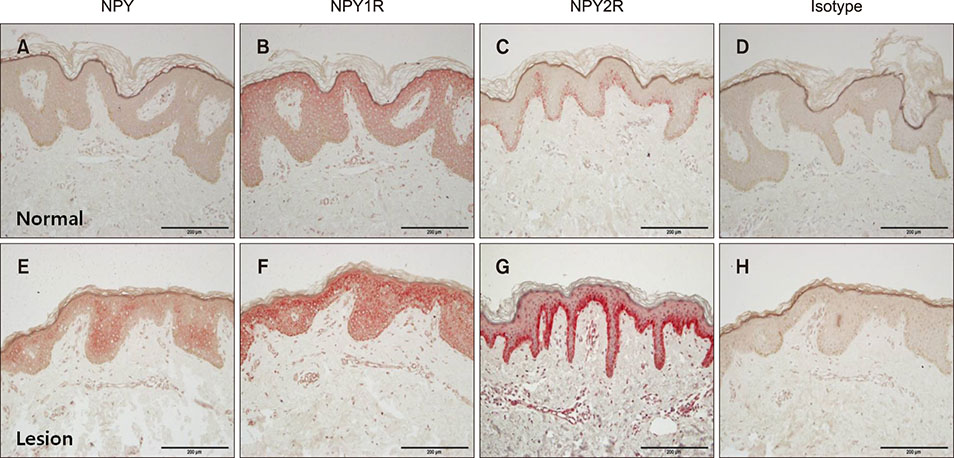
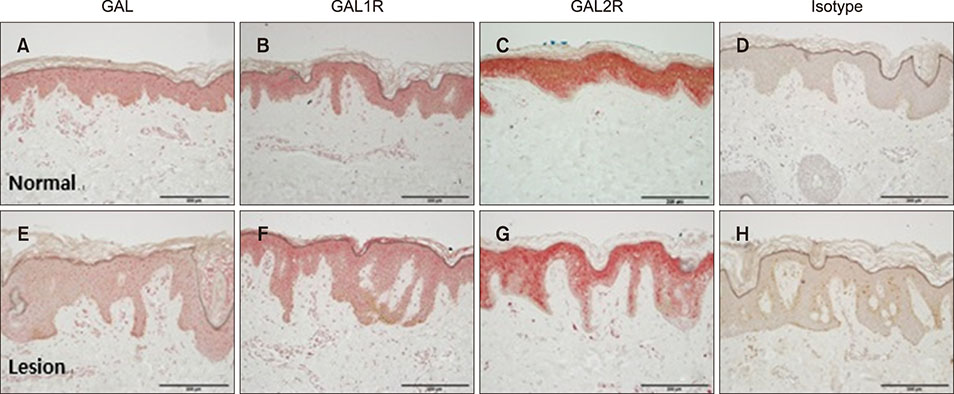
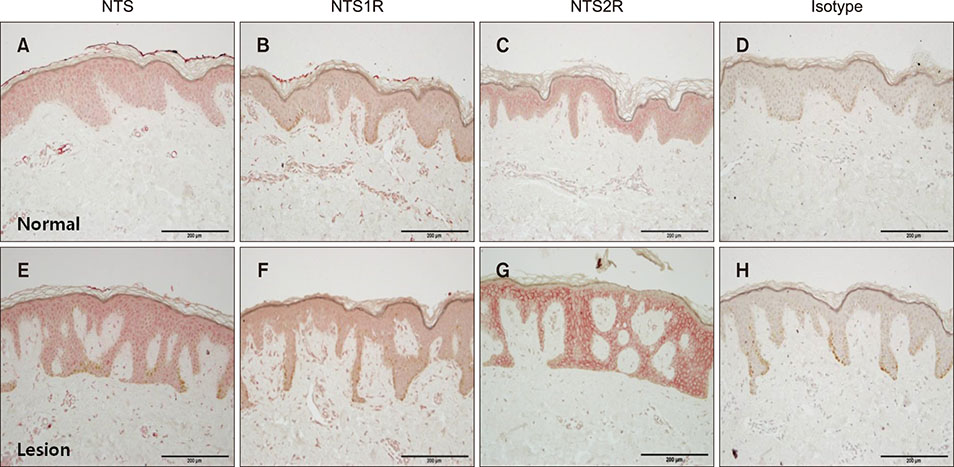
Polymerase chain reaction (PCR) array of 84 genes related to neuronal process was done. Among the genes with 10-fold or more increase in lesional, real-time PCR was performed for neuropeptide Y (NPY), galanin, neurotensin (NTS) and their receptors skin compared to normal skin. IHC stain was done to look for the expression of NPY, galanin, NTS and their receptors and the distribution of protein gene products (PGP) 9.5 immunoreactive nerve fibers.
RESULTS
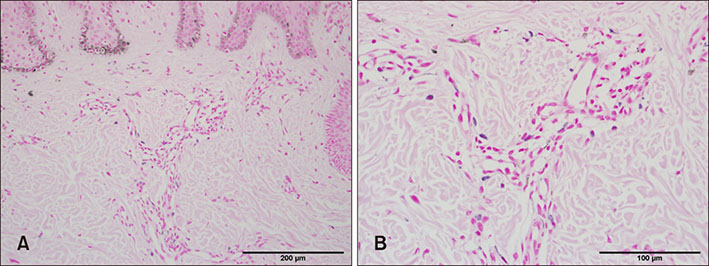
PCR array revealed that 16 out of 84 genes related to neuronal process were increased by 10-fold or more in lesional skin. In real-time PCR of NPY, galanin, NTS and their receptors, statistically significant increase of NPY1R (p < 0.05) and marginally significant increase of NPY2R, GAL2R, and NTS2R (p < 0.1) was verified in lesional skin. In immunohistochemistry, NPY, NPY1R NPY2R, and NTS2R were highly expressed in lesional skin and increased PGP 9.5 immunoreactive linear nerve fibers were found in the epidermis of BN.
CONCLUSION
NPY, galanin, NTS and their receptors and increased innervation may play a role in the pathogenesis of BN.
MeSH Terms
Figure
Reference
-
1. Becker SW. Concurrent melanosis and hypertrichosis in distribution of nevus unius lateris. Arch Derm Syphilol. 1949; 60:155–160.
Article2. Glinick SE, Alper JC, Bogaars H, Brown JA. Becker's melanosis: associated abnormalities. J Am Acad Dermatol. 1983; 9:509–514.
Article3. Person JR, Longcope C. Becker's nevus: an androgen-mediated hyperplasia with increased androgen receptors. J Am Acad Dermatol. 1984; 10:235–238.
Article4. Stanford DG, Georgouras KE. Dermal melanocytosis: a clinical spectrum. Australas J Dermatol. 1996; 37:19–25.
Article5. Zvulunov A, Esterly NB. Neurocutaneous syndromes associated with pigmentary skin lesions. J Am Acad Dermatol. 1995; 32:915–935.
Article6. Hara M, Toyoda M, Yaar M, Bhawan J, Avila EM, Penner IR, et al. Innervation of melanocytes in human skin. J Exp Med. 1996; 184:1385–1395.
Article7. Bousema MT, Vuzevski V, Oranje A, Heule F, Stolz E, van Joost T. Non-von Recklinghausen's neurofibromatosis resembling a giant pigmented nevus. J Am Acad Dermatol. 1989; 20:358–362.
Article8. Zvulunov A, Metzker A. Neurofibromatosis versus giant pigmented nevus. J Am Acad Dermatol. 1990; 23:956–957.
Article9. Bak H, Lee HJ, Chang SE, Choi JH, Kim MN, Kim BJ. Increased expression of nerve growth factor receptor and neural endopeptidase in the lesional skin of melasma. Dermatol Surg. 2009; 35:1244–1250.
Article10. Pahwa P, Sethuraman G. Segmental Becker's nevi with mucosal involvement. Pediatr Dermatol. 2012; 29:670–671.
Article11. Ro YS, Ko JY. Linear congenital Becker nevus. Cutis. 2005; 75:122–124.12. Sheng P, Cheng YL, Cai CC, Guo WJ, Zhou Y, Shi G, et al. Clinicopathological features and immunohistochemical alterations of keratinocyte proliferation, melanocyte density, smooth muscle hyperplasia and nerve fiber distribution in Becker's nevus. Ann Dermatol. 2016; 28:697–703.
Article13. Gilaberte Y, Roca MJ, Garcia-Prats MD, Coscojuela C, Arbues MD, Vera-Alvarez JJ. Neuropeptide Y expression in cutaneous melanoma. J Am Acad Dermatol. 2012; 66:e201–e208.
Article14. Gilaberte Y, Vera J, Coscojuela C, Roca MJ, Parrado C, González S. [Expression of galanin in melanocytic tumors]. Actas Dermosifiliogr. 2007; 98:24–34. Spanish.
Article15. Tomita Y, Maeda K, Tagami H. Mechanisms for hyperpigmentation in postinflammatory pigmentation, urticaria pigmentosa and sunburn. Dermatologica. 1989; 179:Suppl 1. 49–53.
Article16. Nelson JS, Applebaum J. Treatment of superficial cutaneous pigmented lesions by melanin-specific selective photothermolysis using the Q-switched ruby laser. Ann Plast Surg. 1992; 29:231–237.
Article17. Goldberg DJ. Benign pigmented lesions of the skin. Treatment with the Q-switched ruby laser. J Dermatol Surg Oncol. 1993; 19:376–379.18. Chapel TA, Tavafoghi V, Mehregan AH, Gagliardi C. Becker's melanosis: an organoid hamartoma. Cutis. 1981; 27:405–406. 41041519. Metin A, Tuncay I, Uğraş S. About the paper "Elephantiasis neuromatosa and Becker's melanosis" (J Dermatol, 26: 396-398, 1999). J Dermatol. 2001; 28:112–113.
Article20. Hara M, Kumasaka K, Tomita Y, Tagami H. Unilateral dermatomal pigmentary dermatosis: a variant dyschromatosis? J Am Acad Dermatol. 1992; 27:763–764.
Article21. Bröcker EB, Magiera H, Herlyn M. Nerve growth and expression of receptors for nerve growth factor in tumors of melanocyte origin. J Invest Dermatol. 1991; 96:662–665.
Article22. Lazarova R, Hristakieva E, Lazarov N, Shani J. Vitiligo-related neuropeptides in nerve fibers of the skin. Arch Physiol Biochem. 2000; 108:262–267.
Article23. Kim SY, Kim MY, Kang H, Kim HO, Park YM. Becker's naevus in a patient with neurofibromatosis. J Eur Acad Dermatol Venereol. 2008; 22:394–395.
Article24. Mahé E, Zeller J, Wechsler J, Wolkenstein P, Revuz J. [Large hairy pigmented spots in neurofibromatosis type 1: an atypical form of neurofibromas]. Ann Dermatol Venereol. 2001; 128:619–621. French.25. Tatemoto K. Neuropeptide Y and its receptor antagonists. Use of an analog mixture-screening strategy. Ann N Y Acad Sci. 1990; 611:1–6.26. Pedrazzini T, Pralong F, Grouzmann E. Neuropeptide Y: the universal soldier. Cell Mol Life Sci. 2003; 60:350–377.
Article27. Scheenen WJ, Jenks BG, Willems PH, Roubos EW. Action of stimulatory and inhibitory alpha-MSH secretagogues on spontaneous calcium oscillations in melanotrope cells of Xenopus laevis. Pflugers Arch. 1994; 427:244–251.
Article28. Weihe E, Hartschuh W. Multiple peptides in cutaneous nerves: regulators under physiological conditions and a pathogenetic role in skin disease. Semin Dermatol. 1988; 7:284–300.29. Takahashi K, Nakanishi S, Imamura S. Direct effects of cutaneous neuropeptides on adenylyl cyclase activity and proliferation in a keratinocyte cell line: stimulation of cyclic AMP formation by CGRP and VIP/PHM, and inhibition by NPY through G protein-coupled receptors. J Invest Dermatol. 1993; 101:646–651.
Article30. Berger A, Santic R, Hauser-Kronberger C, Schilling FH, Kogner P, Ratschek M, et al. Galanin and galanin receptors in human cancers. Neuropeptides. 2005; 39:353–359.
Article31. Grenbäck E, Bjellerup P, Wallerman E, Lundblad L, Anggård A, Ericson K, et al. Galanin in pituitary adenomas. Regul Pept. 2004; 117:127–139.
Article32. Kofler B, Berger A, Santic R, Moritz K, Almer D, Tuechler C, et al. Expression of neuropeptide galanin and galanin receptors in human skin. J Invest Dermatol. 2004; 122:1050–1053.
Article33. Hafner C, Stempfl T, Bäumler W, Hohenleutner U, Landthaler M, Vogt T. Gene expression profiling of melanocytes following Q-Switched Ruby laser irradiation. Dermatology. 2008; 216:6–13.
Article34. Dallos A, Kiss M, Polyánka H, Dobozy A, Kemény L, Husz S. Galanin receptor expression in cultured human keratinocytes and in normal human skin. J Peripher Nerv Syst. 2006; 11:156–164.
Article35. Ji RR, Zhang X, Zhang Q, Dagerlind A, Nilsson S, Wiesenfeld-Hallin Z, et al. Central and peripheral expression of galanin in response to inflammation. Neuroscience. 1995; 68:563–576.
Article36. Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973; 248:6854–6861.
Article37. Dupouy S, Mourra N, Doan VK, Gompel A, Alifano M, Forgez P. The potential use of the neurotensin high affinity receptor 1 as a biomarker for cancer progression and as a component of personalized medicine in selective cancers. Biochimie. 2011; 93:1369–1378.
Article38. Theoharides TC, Stewart JM, Taracanova A, Conti P, Zouboulis CC. Neuroendocrinology of the skin. Rev Endocr Metab Disord. 2016; 17:287–294.
Article39. Alysandratos KD, Asadi S, Angelidou A, Zhang B, Sismanopoulos N, Yang H, et al. Neurotensin and CRH interactions augment human mast cell activation. PLoS One. 2012; 7:e48934.
Article40. Muallem MM, Rubeiz NG. Physiological and biological skin changes in pregnancy. Clin Dermatol. 2006; 24:80–83.
Article41. Peters EM, Ericson ME, Hosoi J, Seiffert K, Hordinsky MK, Ansel JC, et al. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol. 2006; 126:1937–1947.
Article42. Nordlund JJ. Postinflammatory hyperpigmentation. Dermatol Clin. 1988; 6:185–192.
Article43. Maimone D, Cioni C, Rosa S, Macchia G, Aloisi F, Annunziata P. Norepinephrine and vasoactive intestinal peptide induce IL-6 secretion by astrocytes: synergism with IL-1 beta and TNF alpha. J Neuroimmunol. 1993; 47:73–81.
Article44. Gottschall PE, Tatsuno I, Arimura A. Regulation of interleukin-6 (IL-6) secretion in primary cultured rat astrocytes: synergism of interleukin-1 (IL-1) and pituitary adenylate cyclase activating polypeptide (PACAP). Brain Res. 1994; 637:197–203.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Becker's Nevus Associated with Ipsilateral Hypoplasia
- Becker's Nevus with Recurrent Eczema Limited to the Nevus Lesion
- Two Cases of Backer' s Nevus
- Becker's Nevus Syndrome with Ipsilateral Limb Hyperplasia
- A Case of Becker Nevus Syndrome with a Tooth Defect andFibrous Dysplasia of the Sphenoid Bone