Ann Lab Med.
2019 Jul;39(4):381-387. 10.3343/alm.2019.39.4.381.
Comparison of Six Automated Immunoassays With Isotope-Diluted Liquid Chromatography-Tandem Mass Spectrometry for Total Thyroxine Measurement
- Affiliations
-
- 1Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China. lingqiubj@163.com
- 2National Center for Clinical Laboratories, Beijing Hospital, National Center for Gerontology, Beijing Engineering Research Center of Laboratory Medicine, Beijing, China.
- 3Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, China.
- KMID: 2438885
- DOI: http://doi.org/10.3343/alm.2019.39.4.381
Abstract
- BACKGROUND
Accurate serum total thyroxine (TT4) measurement is important for thyroid disorder diagnosis and management. We compared the performance of six automated immunoassays with that of isotope-diluted liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) as the reference method. We also evaluated the correlation of thyroid stimulating hormone (TSH) with TT4 measured by ID-LC-MS/MS and immunoassays.
METHODS
Serum was collected from 156 patients between October 2015 and January 2016. TT4 was measured by immunoassays from Abbott (Architect), Siemens (ADVIA Centaur XP), Roche (E601), Beckman-Coulter (Dxi800), Autobio (Autolumo A2000), and Mindray (CL-1000i), and by ID-LC-MS/MS. Results were analyzed using Passing-Bablok regression and Bland-Altman plots. Minimum requirements based on biological variation were as follows: a mean bias of ≤4.5% and total imprecision (CV) of ≤3.7%.
RESULTS
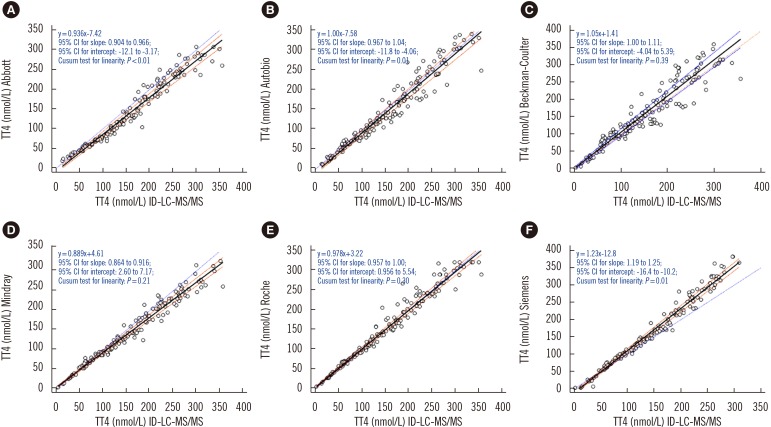
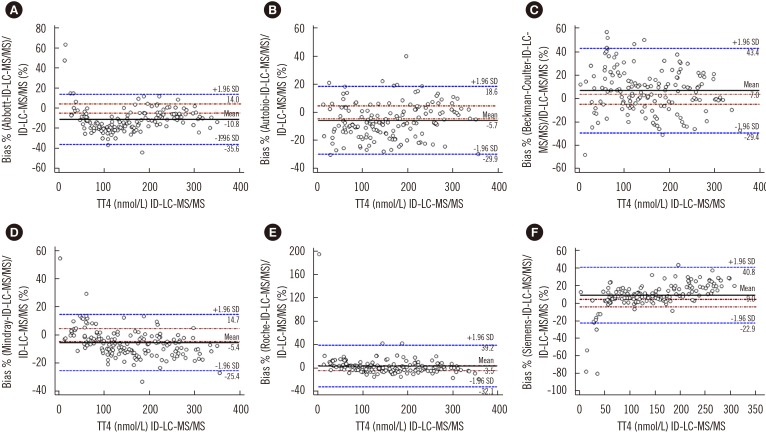
All immunoassays showed a correlation >0.945 with ID-LC-MS/MS; however, the slope of the Passing-Bablok regression line varied from 0.886 (Mindray) to 1.23 (Siemens) and the intercept from −12.8 (Siemens) to 4.61 (Mindray). Only Autobio, Beckman-Coulter, and Roche included the value of one in the 95% confidence interval for slope. The mean bias ranged from −10.8% (Abbott) to 9.0% (Siemens), with the lowest value noted for Roche (3.5%) and the highest for Abbott (−10.8%). Only Abbott and Roche showed within-run and total CV ≤3.7%.
CONCLUSIONS
Though all immunoassays correlated strongly with ID-LC-MS/MS, most did not meet the minimum clinical requirement. Laboratories and immunoassay manufacturers must be aware of these limitations.
Keyword
MeSH Terms
Figure
Reference
-
1. Goemann IM, Romitti M, Meyer ELS, Wajner SM, Maia AL. Role of thyroid hormones in the neoplastic process: an overview. Endocr Relat Cancer. 2017; 24:R367–R385. PMID: 28928142.2. Williams GR, Bassett JHD. Thyroid diseases and bone health. J Endocrinol Invest. 2018; 41:99–109. PMID: 28853052.3. Soh SB, Aw TC. Laboratory testing in thyroid conditions - pitfalls and clinical utility. Ann Lab Med. 2019; 39:3–14. PMID: 30215224.4. Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003; 13:3–126. PMID: 12625976.5. Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989; 10:232–274. PMID: 2673754.6. Thienpont LM, Van Uytfanghe K, Beastall G, Faix JD, Ieiri T, Miller WG, et al. Report of the IFCC Working Group for Standardization of Thyroid Function Tests; Part 2: Free thyroxine and free triiodothyronine. Clin Chem. 2010; 56:912–920. PMID: 20395623.7. Després N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998; 44:440–454. PMID: 9510847.8. Thienpont LM, Van Uytfanghe K, Beastall G, Faix JD, Ieiri T, Miller WG, et al. Report of the IFCC Working Group for Standardization of Thyroid Function Tests; Part 3: Total thyroxine and total triiodothyronine. Clin Chem. 2010; 56:921–929. PMID: 20395622.9. Steele BW, Wang E, Klee GG, Thienpont LM, Soldin SJ, Sokoll LJ, et al. Analytic bias of thyroid function tests: analysis of a College of American Pathologists fresh frozen serum pool by 3900 clinical laboratories. Arch Pathol Lab Med. 2005; 129:310–317. PMID: 15737023.10. Jonklaas J, Sathasivam A, Wang H, Gu J, Burman KD, Soldin SJ. Total and free thyroxine and triiodothyronine: measurement discrepancies, particularly in inpatients. Clin Biochem. 2014; 47:1272–1278. PMID: 24936679.11. Tai SS, Sniegoski LT, Welch MJ. Candidate reference method for total thyroxine in human serum: use of isotope-dilution liquid chromatography-mass spectrometry with electrospray ionization. Clin Chem. 2002; 48:637–642. PMID: 11901062.12. Joint Committee for Traceability in Laboratory Medicine. Database of higher order reference materials, measurement methods/procedures and services. Updated on Oct 2017. https://www.bipm.org/jctlm/.13. Serdar MA, Ozgurtas T, Ispir E, Kenar L, Senes M, Yücel D, et al. Comparison of relationships between FT4 and log TSH in Access DXI 800 Unicel, Modular E170 and ADVIA Centaur XP Analyzer. Clin Chem Lab Med. 2012; 50:1849–1852. PMID: 23089718.14. Jonklaas J, Kahric-Janicic N, Soldin OP, Soldin SJ. Correlations of free thyroid hormones measured by tandem mass spectrometry and immunoassay with thyroid-stimulating hormone across 4 patient populations. Clin Chem. 2009; 55:1380–1388. PMID: 19460839.15. CLSI/NCCLS. User demonstration of performance for precision and trueness; Approved Guideline, NCCLS document EP15-A (ISBN 1-56238-451-1). Wayne, PA: 2001.16. Perich C, Minchinela J, Ricós C, Fernández-Calle P, Alvarez V, Doménech MV, et al. Biological variation database: structure and criteria used for generation and update. Clin Chem Lab Med. 2015; 53:299–305. PMID: 25415636.17. Minchinela J, Ricós C, Perich C, Fernández-Calle P, Alvarez V, Doménech MV, et al. Desirable specifications for total error, imprecision, and bias, derived from intra- and inter-individual biologic variation. Updated on 2014. http://www.westgard.com/biodatabase-2014-update.htm.18. Petersen PH, Fraser CG, Jørgensen L, Brandslund I, Stahl M, Gowans E, et al. Combination of analytical quality specifications based on biological within- and between-subject variation. Ann Clin Biochem. 2002; 39:543–550. PMID: 12564835.19. Bilić-Zulle L. Comparison of methods: Passing and Bablok regression. Biochem Med (Zagreb). 2011; 21:49–52. PMID: 22141206.20. Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb). 2015; 25:141–151. PMID: 26110027.21. Burtis CA, Ashwood ER, et al. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed. Saint Louis, MO: Elsevier;2012. p. 1931–1933.22. Medicare, Medicaid and CLIA programs; Regulations Implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA)-HCFA. Final rule with comment period. Fed Regist. 1992; 57:7002–7186. PMID: 10170937.23. Nelson JC, Wang R, Asher DT, Wilcox RB. Underestimates and overestimates of total thyroxine concentrations caused by unwanted thyroxine-binding protein effects. Thyroid. 2005; 15:12–15. PMID: 15687815.24. Stockigt JR, Lim CF. Medications that distort in vitro tests of thyroid function, with particular reference to estimates of serum free thyroxine. Best Pract Res Clin Endocrinol Metab. 2009; 23:753–767. PMID: 19942151.25. Bowen RA, Sattayapiwat A, Gounden V, Remaley AT. Blood collection tube-related alterations in analyte concentrations in quality control material and serum specimens. Clin Biochem. 2014; 47:150–157. PMID: 24240064.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Accurate Isotope Dilution Liquid Chromatography-Tandem Mass Spectrometry Method for Serum C-Peptide and Its Use in Harmonization in China
- A Sensitive and Specific Liquid ChromatographyTandem Mass Spectrometry Assay for Simultaneous Quantification of Salivary Melatonin and Cortisol: Development and Comparison With Immunoassays
- Performance Evaluation of the ARCHITECT i2000 for the Determination of Whole Blood Cyclosporin A and Tacrolimus
- Comparison of Direct and Extraction Immunoassay Methods With Liquid Chromatography-Tandem Mass Spectrometry Measurement of Urinary Free Cortisol for the Diagnosis of Cushing’s Syndrome
- Development and Validation of a Novel Isotope Dilution-Ultraperformance Liquid ChromatographyTandem Mass Spectrometry Method for Serum C-Peptide