Ann Lab Med.
2024 Jan;44(1):29-37. 10.3343/alm.2024.44.1.29.
Comparison of Direct and Extraction Immunoassay Methods With Liquid Chromatography-Tandem Mass Spectrometry Measurement of Urinary Free Cortisol for the Diagnosis of Cushing’s Syndrome
- Affiliations
-
- 1Department of Laboratory Medicine, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- 2State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- 3Department of Endocrinology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- KMID: 2550220
- DOI: http://doi.org/10.3343/alm.2024.44.1.29
Abstract
- Background
Twenty-four-hour urinary free cortisol (UFC) measurement is the initial diagnostic test for Cushing’s syndrome (CS). We compared UFC determination by both direct and extraction immunoassays using Abbott Architect, Siemens Atellica Solution, and Beckman DxI800 with liquid chromatography-tandem mass spectrometry (LC-MS/MS). In addition, we evaluated the value of 24-hr UFC measured by six methods for diagnosing CS.
Methods
Residual 24-hr urine samples of 94 CS and 246 non-CS patients were collected. A laboratory-developed LC-MS/MS method was used as reference. UFC was measured by direct assays (D) using Abbott, Siemens, and Beckman platforms and by extraction assays (E) using Siemens and Beckman platforms. Method was compared using Passing–Bablok regression and Bland–Altman plot analyses. Cut-off values for the six assays and corresponding sensitivities and specificities were calculated by ROC analysis.
Results
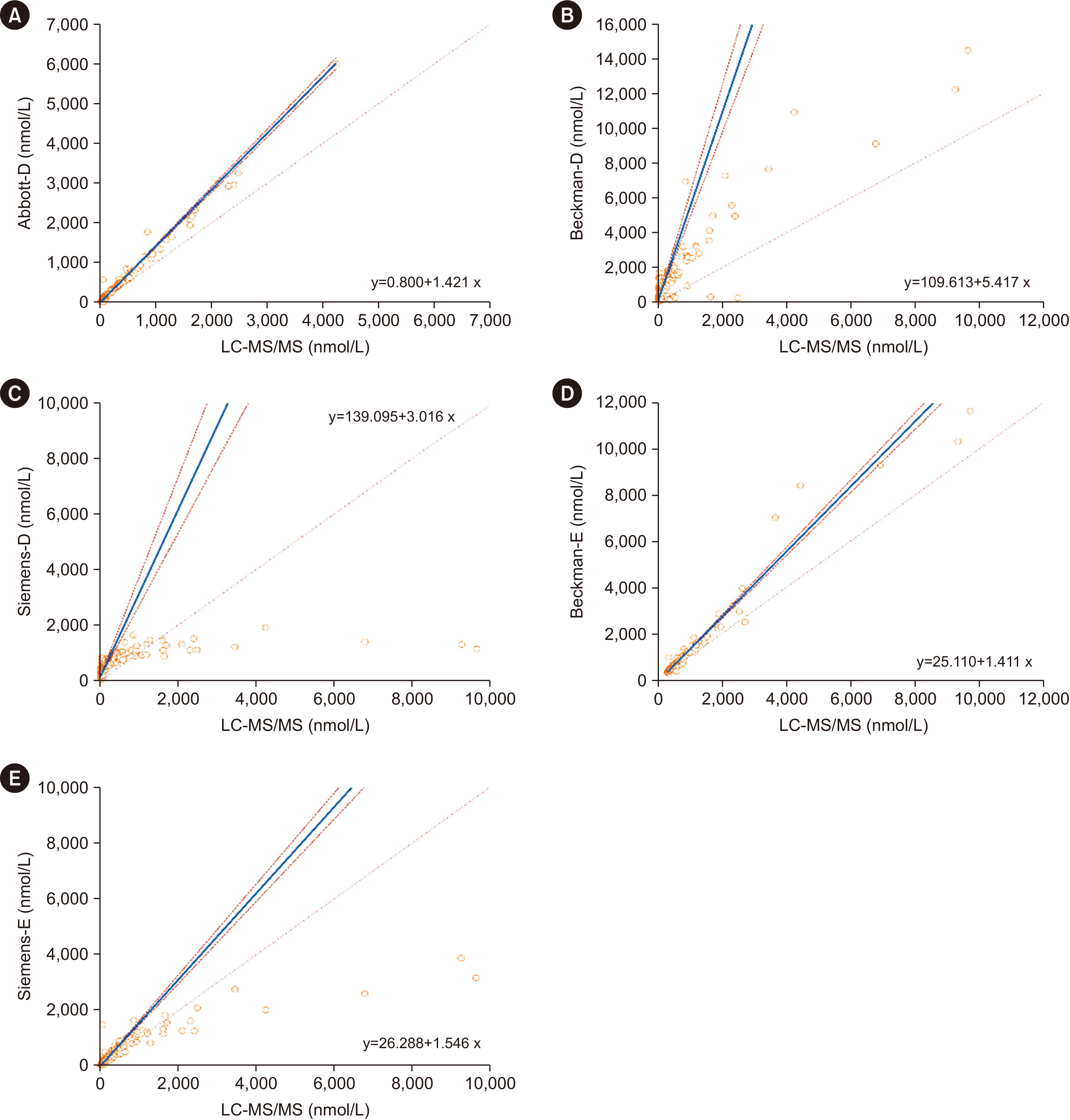
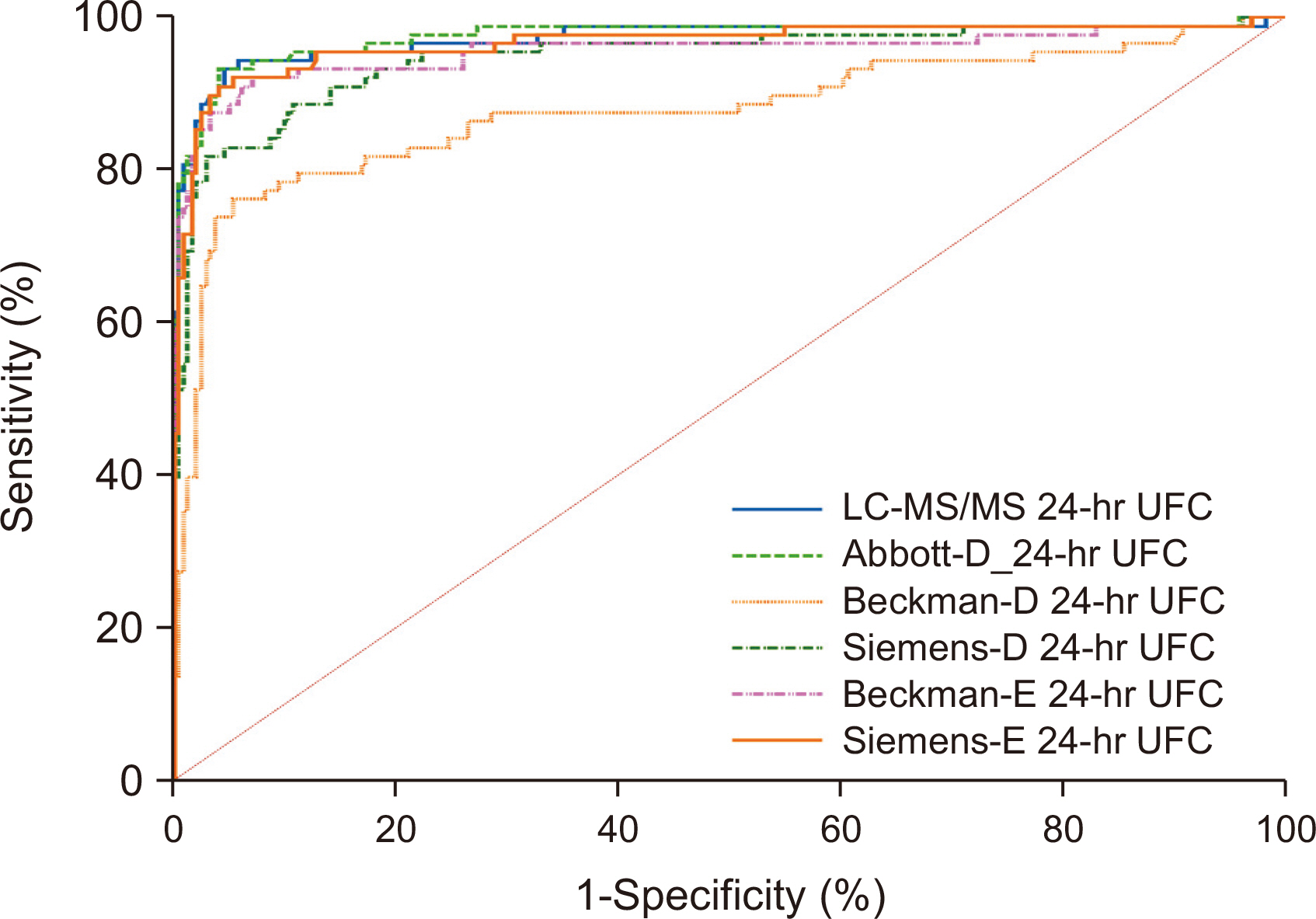
Abbott-D, Beckman-E, Siemens-E, and Siemens-D showed strong correlations with LC-MS/MS (Spearman coefficient r = 0.965, 0.922, 0.922, and 0.897, respectively), while Beckman-D showed weaker correlation (r = 0.755). All immunoassays showed proportionally positive bias. The areas under the curve were 0.975 for Abbott-D, 0.972 for LCMS/MS, 0.966 for Siemens-E, 0.948 for Siemens-D, 0.955 for Beckman-E, and 0.877 for Beckman-D. The cut-off values varied significantly (154.8–1,321.5 nmol/24 hrs). Assay sensitivity and specificity ranged from 76.1% to 93.2% and from 93.0% to 97.1%, respectively.
Conclusions
Commercially available immunoassays for measuring UFC show different levels of analytical consistency compared to LC-MS/MS. Abbott-D, Siemens-E, and Beckman-E have high diagnostic accuracy for CS.
Keyword
Figure
Reference
-
1. Raff H, Findling JW. 2003; A physiologic approach to diagnosis of the Cushing syndrome. Ann Intern Med. 138:980–91. DOI: 10.7326/0003-4819-138-12-200306170-00010. PMID: 12809455.
Article2. El-Farhan N, Rees DA, Evans C. 2017; Measuring cortisol in serum, urine and saliva - are our assays good enough? Ann Clin Biochem. 54:308–22. DOI: 10.1177/0004563216687335. PMID: 28068807.
Article3. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. 2008; The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 93:1526–40. DOI: 10.1210/jc.2008-0125. PMID: 18334580. PMCID: PMC2386281.
Article4. Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, et al. 2003; Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 88:5593–602. DOI: 10.1210/jc.2003-030871. PMID: 14671138.
Article5. Pivonello R, De Leo M, Cozzolino A, Colao A. 2015; The treatment of Cushing's disease. Endocr Rev. 36:385–486. DOI: 10.1210/er.2013-1048. PMID: 26067718. PMCID: PMC4523083.
Article6. Ray JA, Bajaj AO, Trier EK, Johnson LM. 2022; Iatrogenic Cushing syndrome in 24-hour urine free cortisol measurement. Clin Chim Acta. 534:173–5. DOI: 10.1016/j.cca.2022.07.019.
Article7. Galm BP, Qiao N, Klibanski A, Biller BMK, Tritos NA. Accuracy of laboratory tests for the diagnosis of Cushing syndrome. J Clin Endocrinol Metab. 2020; 105:DOI: 10.1210/clinem/dgaa105. PMID: 32133504.
Article8. Turpeinen U, Hämäläinen E. 2013; Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab. 27:795–801. DOI: 10.1016/j.beem.2013.10.008. PMID: 24275191.
Article9. La'ulu SL, Roberts WL. 2008; Performance characteristics of the Architect cortisol immunoassay. Clin Chim Acta. 388:219–21. DOI: 10.1016/j.cca.2007.11.005. PMID: 18062923.10. Wood L, Ducroq DH, Fraser HL, Gillingwater S, Evans C, Pickett AJ, et al. 2008; Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography-mass spectrometry and two commercial immunoassays. Ann Clin Biochem. 45:380–8. DOI: 10.1258/acb.2007.007119.
Article11. Raff H, Auchus RJ, Findling JW, Nieman LK. 2015; Urine free cortisol in the diagnosis of Cushing's syndrome: is it worth doing and, if so, how? J Clin Endocrinol Metab. 100:395–7. DOI: 10.1210/jc.2014-3766. PMID: 25423573. PMCID: PMC4318888.
Article12. Bianchi L, Campi B, Sessa MR, De Marco G, Ferrarini E, Zucchi R, et al. 2019; Measurement of urinary free cortisol by LC-MS-MS: adoption of a literature reference range and comparison with our current immunometric method. J Endocrinol Invest. 42:1299–305. DOI: 10.1007/s40618-019-01050-5. PMID: 31012054.
Article13. Konforte D, Bozovic A, Mah W, Martens P, Yip PM. 2011; Validation of the cortisol method on the Abbott architect® i2000 immunoassay system for measurement of urine cortisol by comparison to LC-MS-MS. Clin Biochem. 44:1175. DOI: 10.1016/j.clinbiochem.2011.06.040.
Article14. Luo A, El Gierari ETM, Nally LM, Sturmer LR, Dodd D, Shi RZ. 2019; Clinical utility of an ultrasensitive urinary free cortisol assay by tandem mass spectrometry. Steroids. 146:65–9. DOI: 10.1016/j.steroids.2019.03.014. PMID: 30951757.
Article15. Oßwald A, Wang R, Beuschlein F, Hartmann MF, Wudy SA, Bidlingmaier M, et al. 2019; Performance of LC-MS/MS and immunoassay based 24-h urine free cortisol in the diagnosis of Cushing's syndrome. J Steroid Biochem Mol Biol. 190:193–7. DOI: 10.1016/j.jsbmb.2019.04.004. PMID: 30959155.
Article16. Ceccato F, Barbot M, Zilio M, Frigo AC, Albiger N, Camozzi V, et al. 2015; Screening tests for Cushing's syndrome: urinary free cortisol role measured by LC-MS/MS. J Clin Endocrinol Metab. 100:3856–61. DOI: 10.1210/jc.2015-2507. PMID: 26274344.
Article17. Aranda G, Careaga M, Hanzu FA, Patrascioiu I, Ríos P, Mora M, et al. 2016; Accuracy of immunoassay and mass spectrometry urinary free cortisol in the diagnosis of Cushing's syndrome. Pituitary. 19:496–502. DOI: 10.1007/s11102-016-0730-5. PMID: 27259502.
Article18. CLSI. 2014. User verification of precision and estimation of bias; approved guideline. 3rd ed. EP15-A3. Clinical and Laboratory Standards Institute;Wayne, PA:19. Perrin P, Plotton I, Berthiller J, Borson-Chazot F, Raverot G, Raverot V. 2020; Urinary free cortisol: an automated immunoassay without extraction for diagnosis of Cushing's syndrome and follow-up of patients treated by anticortisolic drugs. Clin Endocrinol (Oxf). 93:76–8. DOI: 10.1111/cen.14200. PMID: 32306407.20. Horie H, Kidowaki T, Koyama Y, Endo T, Homma K, Kambegawa A, et al. 2007; Specificity assessment of immunoassay kits for determination of urinary free cortisol concentrations. Clin Chim Acta. 378:66–70. DOI: 10.1016/j.cca.2006.10.018. PMID: 17174290.
Article21. Wudy SA, Schuler G, Sánchez-Guijo A, Hartmann MF. 2018; The art of measuring steroids: principles and practice of current hormonal steroid analysis. J Steroid Biochem Mol Biol. 179:88–103. DOI: 10.1016/j.jsbmb.2017.09.003.22. Ceccato F, Antonelli G, Barbot M, Zilio M, Mazzai L, Gatti R, et al. 2014; The diagnostic performance of urinary free cortisol is better than the cortisol:cortisone ratio in detecting de novo Cushing's syndrome: the use of a LC-MS/MS method in routine clinical practice. Eur J Endocrinol. 171:1–7. DOI: 10.1530/EJE-14-0061. PMID: 24743401.
Article23. Sheriff N, McCormack AI. 2017; How useful is urinary-free cortisol in the clinic? Biomark Med. 11:1009–16. DOI: 10.2217/bmm-2016-0311. PMID: 29039221.
Article24. Kotłowska A, Puzyn T, Sworczak K, Stepnowski P, Szefer P. 2017; Metabolomic biomarkers in urine of Cushing's syndrome patients. Int J Mol Sci. 18:294. DOI: 10.3390/ijms18020294. PMID: 28146078. PMCID: PMC5343830.
Article25. Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Sen K, et al. 2014; High variability in baseline urinary free cortisol values in patients with Cushing's disease. Clin Endocrinol (Oxf). 80:261–9. DOI: 10.1111/cen.12259. PMID: 23746264. PMCID: PMC4231220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Sensitive and Specific Liquid ChromatographyTandem Mass Spectrometry Assay for Simultaneous Quantification of Salivary Melatonin and Cortisol: Development and Comparison With Immunoassays
- Cortisol Measurements in Cushing's Syndrome: Immunoassay or Mass Spectrometry?
- Clinical and Technical Aspects in Free Cortisol Measurement
- Usefullness of Urinary Free Cortisol Measurement in Diagnosis of Iatrogenic Cushing Syndrome
- Dried Blood Spot Multiplexed Steroid Profiling Using Liquid Chromatography Tandem Mass Spectrometry in Korean Neonates