Anesth Pain Med.
2018 Apr;13(2):122-127. 10.17085/apm.2018.13.2.122.
Updated review of resistance to neuromuscular blocking agents
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chosun University Hospital, Chosun University School of Medicine, Gwangju, Korea. than@chosun.ac.kr
- KMID: 2437417
- DOI: http://doi.org/10.17085/apm.2018.13.2.122
Abstract
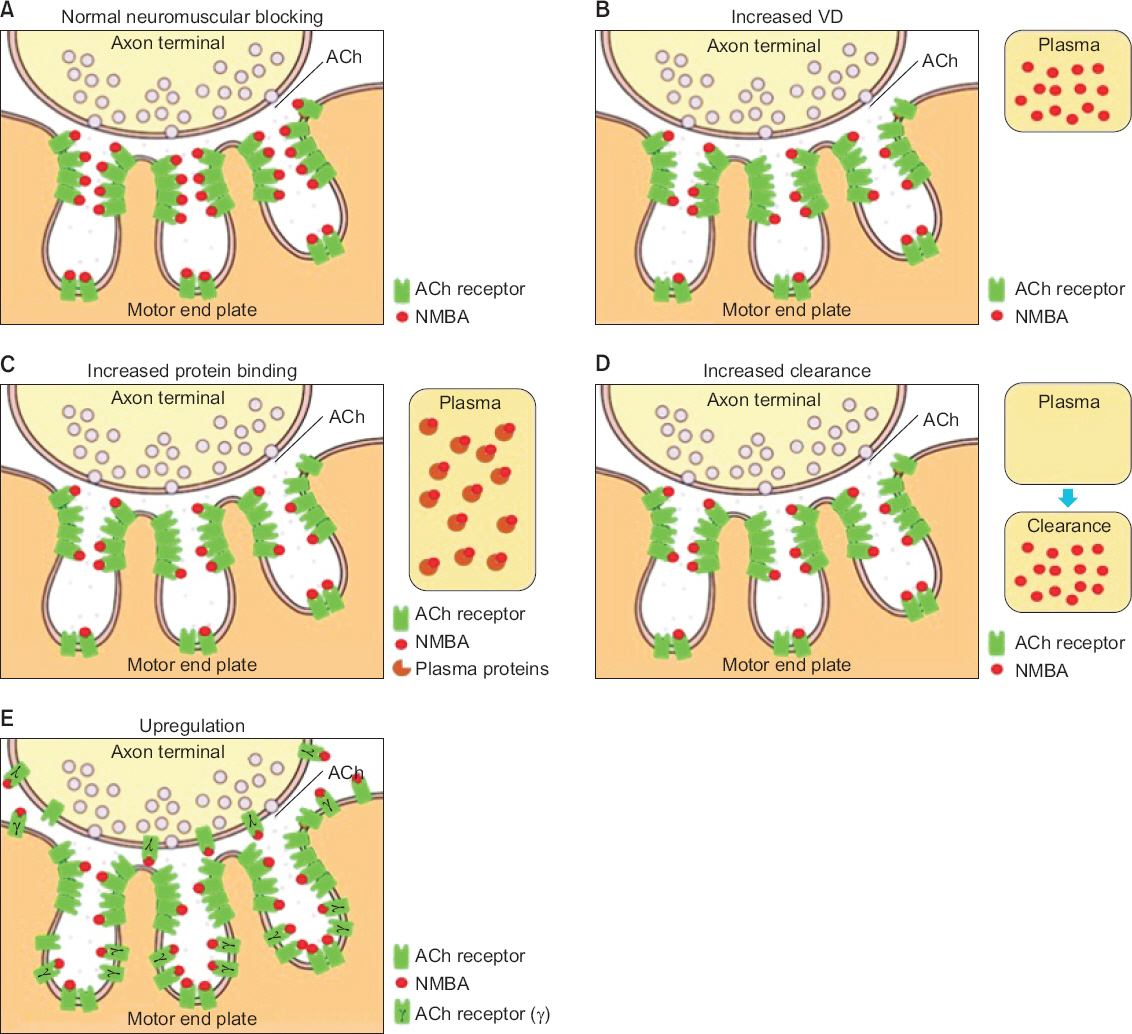
- Since neuromuscular blocking agents (NMBAs) were introduced to the surgical field, they have become almost mandatory for the induction and maintenance of anesthesia. However, resistance to NMBAs can develop in certain pathological states, such as central nerve injury, burns, and critical illnesses. During such pathological processes, quantitative and qualitative changes occur in the physiology of acetylcholine and the acetylcholine receptor (AChR) at the neuromuscular junction. Up-regulation of AChR leads to changes in the pharmacokinetics and pharmacodynamics of NMBA. As NMBA resistance may result in problems during anesthesia, it is of utmost importance to understand the mechanisms of NMBA resistance and their associations with pathological status to maintain adequate neuromuscular relaxation. This review presents the current knowledge of pharmacokinetic and pharmacodynamic changes and pathological status associated with NMBA resistance.
MeSH Terms
Figure
Cited by 1 articles
-
Neuromuscular monitoring of a patient with Charcot-Marie-Tooth disease; which monitoring technique is adequate? - A case report and literature review -
Seung Un Kim, Seora Kim, Ki Tae Jung
Anesth Pain Med. 2024;19(1):54-61. doi: 10.17085/apm.23111.
Reference
-
1. Tschida SJ, Graupe KJ, Hoey LL, Vance-Bryan K. Resistance to nondepolarizing neuromuscular blocking agents. Pharmacotherapy. 1996; 16:409–18. PMID: 8726599.2. Martyn JA, White DA, Gronert GA, Jaffe RS, Ward JM. Up-and-down regulation of skeletal muscle acetylcholine receptors. Effects on neuromuscular blockers. Anesthesiology. 1992; 76:822–43. DOI: 10.1097/00000542-199205000-00022. PMID: 1575351.3. Dubovoy T, Shanks AM, Devine S, Kheterpal S. Frequency of inadequate neuromuscular blockade during general anesthesia. J Clin Anesth. 2017; 36:16–20. DOI: 10.1016/j.jclinane.2016.09.020. PMID: 28183558.4. Moorthy SS, Hilgenberg JC. Resistance to non-depolarizing muscle relaxants in paretic upper extremities of patients with residual hemiplegia. Anesth Analg. 1980; 59:624–7. DOI: 10.1213/00000539-198008000-00011. PMID: 7190801.5. Marathe PH, Dwersteg JF, Pavlin EG, Haschke RH, Heimbach DM, Slattery JT. Effect of thermal injury on the pharmacokinetics and pharmacodynamics of atracurium in humans. Anesthesiology. 1989; 70:752–5. DOI: 10.1097/00000542-198905000-00007. PMID: 2719307.6. Fink H, Luppa P, Mayer B, Rosenbrock H, Metzger J, Martyn JA, et al. Systemic inflammation leads to resistance to atracurium without increasing membrane expression of acetylcholine receptors. Anesthesiology. 2003; 98:82–8. DOI: 10.1097/00000542-200301000-00016. PMID: 12502983.7. Parker CJ, Hunter JM. Pharmacokinetics of atracurium and laudanosine in patients with hepatic cirrhosis. Br J Anaesth. 1989; 62:177–83. DOI: 10.1093/bja/62.2.177.8. Leibel WS, Martyn JA, Szyfelbein SK, Miller KW. Elevated plasma binding cannot account for the burn-related d-tubocurarine hyposensitivity. Anesthesiology. 1981; 54:378–82. DOI: 10.1097/00000542-198105000-00006. PMID: 7224206.9. Tatman AJ, Wrigley SR, Jones RM. Resistance to atracurium in a patient with an increase in plasma alpha 1 globulins. Br J Anaesth. 1991; 67:623–5. DOI: 10.1093/bja/67.5.623. PMID: 1721517.10. Lüleci N, Aktürk G, Kalaç N. Resistance to atracurium: wegener's granulomatosis--a case report. Middle East J Anaesthesiol. 1994; 12:511–5. PMID: 7523845.11. Yam CI, Wood P. Repeated resistance to non-depolarizing neuromuscular blocking drugs in a patient with multiple myeloma. Br J Anaesth. 1992; 69:111. DOI: 10.1093/bja/69.1.111.12. Anderson GD. A mechanistic approach to antiepileptic drug interactions. Ann Pharmacother. 1998; 32:554–63. DOI: 10.1345/aph.17332. PMID: 9606477.13. Martyn J, Goldhill DR, Goudsouzian NG. Clinical pharmacology of muscle relaxants in patients with burns. J Clin Pharmacol. 1986; 26:680–5. DOI: 10.1002/j.1552-4604.1986.tb02972.x. PMID: 2947935.14. de Barcelos CC, Braga Ade F, Braga FS, Potério GB, Fernandes SC, Franco YO, et al. In vitro and in vivo neuromuscular effects of atracurium and rocuronium in rats treated with carbamazepine for seven days. Rev Bras Anestesiol. 2008; 58:137–51. PMID: 19378532.15. Hunter JM. Resistance to non-depolarizing neuromuscular blocking agents. Br J Anaesth. 1991; 67:511–4. DOI: 10.1093/bja/67.5.511. PMID: 1836340.16. Gronert GA, Matteo RS, Perkins S. Canine gastrocnemius disuse atrophy: resistance to paralysis by dimethyl tubocurarine. J Appl Physiol Respir Environ Exerc Physiol. 1984; 57:1502–6. DOI: 10.1152/jappl.1984.57.5.1502.17. Maclagan J, Vrbová G. A study of the increased sensitivity of denervated and re-innervated muscle to depolarizing drugs. J Physiol. 1966; 182:131–43. DOI: 10.1113/jphysiol.1966.sp007813. PMID: 5937407. PMCID: PMC1357460.18. Ziskind L, Dennis MJ. Depolarising effect of curare on embryonic rat muscles. Nature. 1978; 276:622–3. DOI: 10.1038/276622a0. PMID: 723945.19. Fambrough DM. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979; 59:165–227. DOI: 10.1152/physrev.1979.59.1.165. PMID: 375254.20. Hogue CW Jr, Itani MS, Martyn JA. Resistance to d-tubocurarine in lower motor neuron injury is related to increased acetylcholine receptors at the neuromuscular junction. Anesthesiology. 1990; 73:703–9. DOI: 10.1097/00000542-199010000-00016.21. Iwasaki H, Namiki A, Omote K, Omote T, Takahashi T. Response differences of paretic and healthy extremities to pancuronium and neostigmine in hemiplegic patients. Anesth Analg. 1985; 64:864–6. DOI: 10.1213/00000539-198509000-00004. PMID: 4025852.22. Benecke R, Berthold A, Conrad B. Denervation activity in the EMG of patients with upper motor neuron lesions: time course, local distribution and pathogenetic aspects. J Neurol. 1983; 230:143–51. DOI: 10.1007/BF00313625. PMID: 6197509.23. Pop PH, Notermans SL, De Graaf R. Muscular denervation in lesions of the central nervous system and its correlation with motor function. Acta Neurol (Napoli). 1988; 10:93–7.24. Tamam Y, Tamam C, Tamam B, Ustundag M, Orak M, Tasdemir N. Peripheral neuropathy after burn injury. Eur Rev Med Pharmacol Sci. 2013; 17(Suppl1):107–11. PMID: 23436672.25. Kim C, Martyn J, Fuke N. Burn injury to trunk of rat causes denervation-like responses in the gastrocnemius muscle. J Appl Physiol (1985). 1988; 65:1745–51. DOI: 10.1152/jappl.1988.65.4.1745. PMID: 3182535.26. Martyn J. Clinical pharmacology and drug therapy in the burned patient. Anesthesiology. 1986; 65:67–75. DOI: 10.1097/00000542-198607000-00011. PMID: 2873763.27. Kim C, Fuke N, Martyn JA. Burn injury to rat increases nicotinic acetylcholine receptors in the diaphragm. Anesthesiology. 1988; 68:401–6. DOI: 10.1097/00000542-198803000-00014. PMID: 3344995.28. Gronert GA. Disuse atrophy with resistance to pancuronium. Anesthesiology. 1981; 55:547–9. DOI: 10.1097/00000542-198111000-00012. PMID: 7294410.29. Berg DK, Hall ZW. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975; 244:659–76. DOI: 10.1113/jphysiol.1975.sp010818.30. Gray HS, Slater RM, Pollard BJ. The effect of acutely administered phenytoin on vecuronium-induced neuromuscular blockade. Anaesthesia. 1989; 44:379–81. DOI: 10.1111/j.1365-2044.1989.tb11331.x. PMID: 2568098.31. Simpson LL. Molecular pharmacology of botulinum toxin and tetanus toxin. Annu Rev Pharmacol Toxicol. 1986; 26:427–53. DOI: 10.1146/annurev.pa.26.040186.002235. PMID: 3521461.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuromuscular Dysfunction in Patients Using Neuromuscular Blocking Agents
- Tracheal intubation without neuromuscular blocking agents
- Multiple cross-reactivity to several types of neuromuscular blocking agents in a patient with rocuronium anaphylaxis
- The Relationships between Onset Time and Recovery of Six Neuromuscular Blocking Drugs
- Neuromuscular Blocking Action of the Aminoglycoside Antibiotics Sisomycin and Micronomycin in the Rabbit