Acute Crit Care.
2018 Aug;33(3):178-184. 10.4266/acc.2017.00444.
Specification of Subject Sex in Oncology-Related Animal Studies
- Affiliations
-
- 1Korea University College of Medicine, Seoul, Korea.
- 2Department of Anesthesiology and Pain Medicine, Korea University Anam Hospital, Seoul, Korea. yourejoice@korea.ac.kr
- 3Department of Thoracic and Cardiovascular Surgery, Korea University Anam Hospital, Seoul, Korea.
- 4Osong Medical Innovation Foundation, Cheongju, Korea.
- KMID: 2436330
- DOI: http://doi.org/10.4266/acc.2017.00444
Abstract
- BACKGROUND
Growing evidence for clinically significant differences between the sexes has attracted the attention of researchers. However, failures to report a test animal sex and balance the sex ratios of study samples remain widespread in preclinical investigations. We analyzed the sex-reporting rate and sex distributions of test animals in published oncology studies.
METHODS
We selected five oncology journals included in the Scientific Citation Index (SCI) based on impact factors. We identified preclinical investigations with in vivo mouse experiments published in 2015 for inclusion in our study sample. We classified each article by whether or not it reported test subject sex, and by which sex was included. We also recorded whether there were justifications for using one particular sex in single-sex studies (e.g., anatomical reasons) and whether sex-based analyses were conducted for both-sex studies.
RESULTS
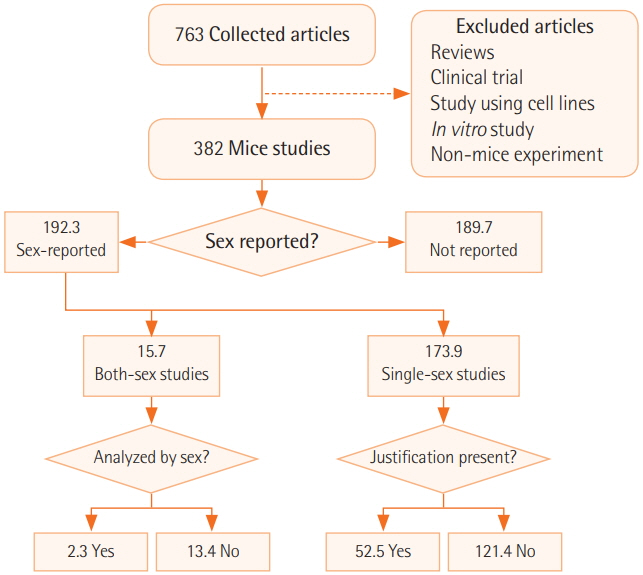
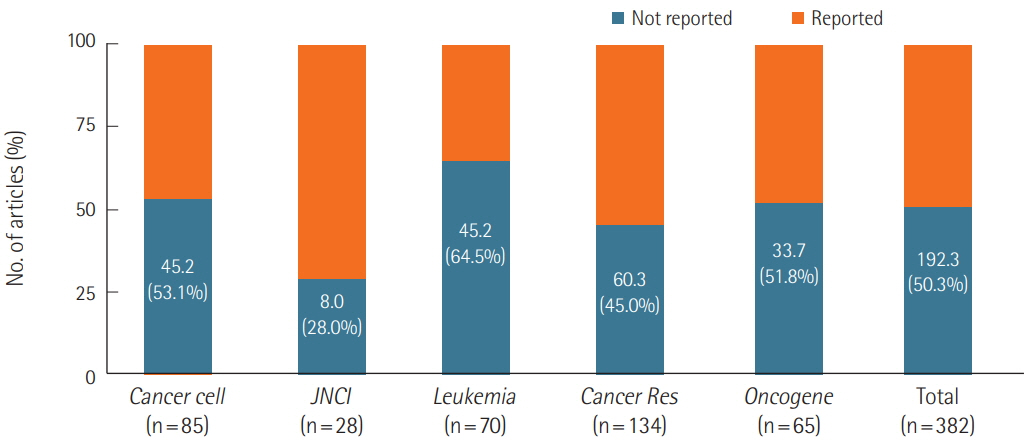
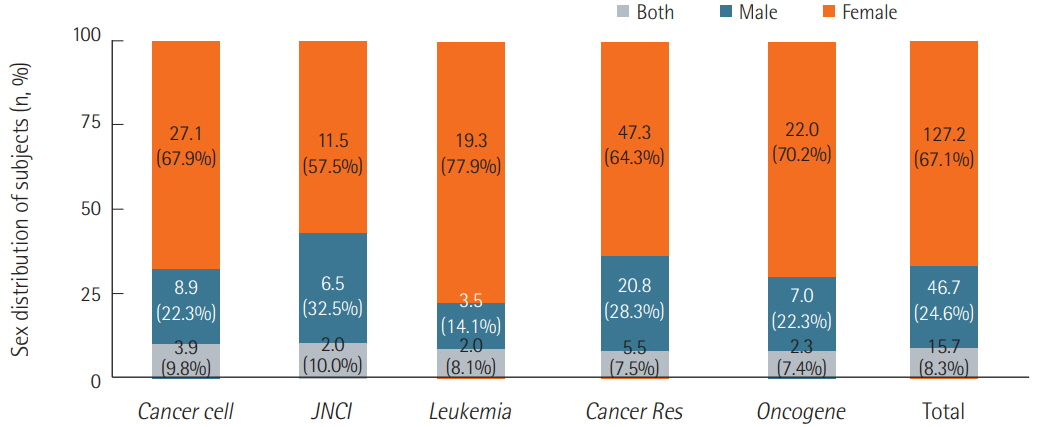
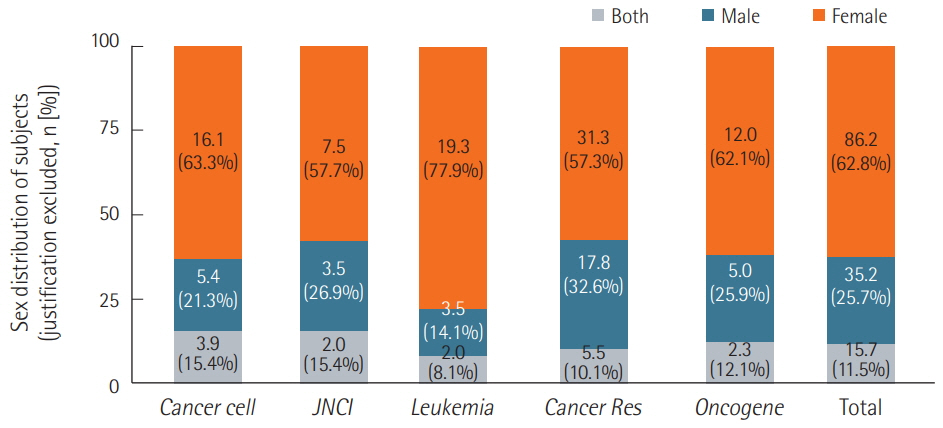
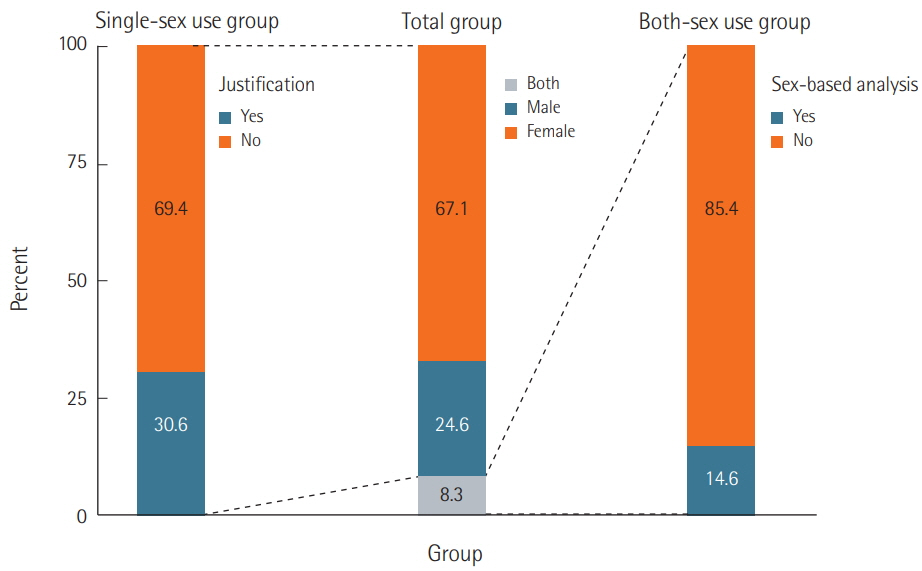
We surveyed a total of 382 articles. Half (50.3%) failed to report test animal sex. Among articles that did report sex, 91.7% were single-sex studies, of which 69.4% did not provide any justifications for using the sex included in the study. Relatively few studies 15.7 studies included animals of both sexes, and only 2.3 studies conducted sex-based analyses. These findings are consistent with those of previous research that used other methods to collect data from the literature such as text mining, but our analysis of the provision of justifications for using one sex versus the other is a novel feature.
CONCLUSIONS
Many researchers overlook test subject sex as a factor, but test animal sex should be reported in all preclinical investigations to enhance the reproducibility of research and avoid faulty conclusions drawn from one-sided studies.
Keyword
MeSH Terms
Figure
Reference
-
1. Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007; 55:81–95.
Article2. Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, et al. Gender differences in pressure-natriuresis and renal autoregulation: role of the angiotensin type 2 receptor. Hypertension. 2011; 57:275–82.3. Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab. 2011; 96:885–93.
Article4. Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, et al. A role for cell sex in stem cellmediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007; 177:73–86.
Article5. Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004; 279:38563–70.
Article6. Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015; 18:1081–3.
Article7. Sechzer JA, Rabinowitz VC, Denmark FL, McGinn MF, Weeks BM, Wilkens CL. Sex and gender bias in animal research and in clinical studies of cancer, cardiovascular disease, and depression. Ann N Y Acad Sci. 1994; 736:21–48.
Article8. Huang RS, Kistner EO, Bleibel WK, Shukla SJ, Dolan ME. Effect of population and gender on chemotherapeutic agentinduced cytotoxicity. Mol Cancer Ther. 2007; 6:31–6.
Article9. Midgley R, Kerr DJ. Capecitabine: have we got the dose right? Nat Clin Pract Oncol. 2009; 6:17–24.
Article10. Wang J, Huang Y. Pharmacogenomics of sex difference in chemotherapeutic toxicity. Curr Drug Discov Technol. 2007; 4:59–68.11. Begley CG, Ellis LM. Drug development: raise standards for preclinical cancer research. Nature. 2012; 483:531–3.12. Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011; 35:565–72.
Article13. Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005; 117:1–5.
Article14. Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, et al. Feedback suppression of PI3Kα signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kα. Cancer Cell. 2015; 27:109–22.
Article15. Langenkamp E, Zhang L, Lugano R, Huang H, Elhassan TE, Georganaki M, et al. Elevated expression of the C-type lectin CD93 in the glioblastoma vasculature regulates cytoskeletal rearrangements that enhance vessel function and reduce host survival. Cancer Res. 2015; 75:4504–16.
Article16. Adams GN, Rosenfeldt L, Frederick M, Miller W, Waltz D, Kombrinck K, et al. Colon cancer growth and dissemination relies upon thrombin, stromal PAR-1, and fibrinogen. Cancer Res. 2015; 75:4235–43.
Article17. Wang SH, Yeh SH, Shiau CW, Chen KF, Lin WH, Tsai TF, et al. Sorafenib action in hepatitis B virus X-activated oncogenic androgen pathway in liver through SHP-1. J Natl Cancer Inst. 2015; 107:pii: djv190.
Article18. Kim T, Jeon YJ, Cui R, Lee JH, Peng Y, Kim SH, et al. Role of MYC-regulated long noncoding RNAs in cell cycle regulation and tumorigenesis. J Natl Cancer Inst. 2015; 107:pii: dju505.
Article19. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8:e1000412.
Article20. Flórez-Vargas O, Brass A, Karystianis G, Bramhall M, Stevens R, Cruickshank S, et al. Bias in the reporting of sex and age in biomedical research on mouse models. Elife. 2016; 5:pii: e13615.
Article21. McCullough LD, de Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Differ. 2014; 5:15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Smoking Related Social Influence, Refusal Skill and Nonsmoking Related Self-efficacy among Adolescents
- Experimental Models of Depression
- A Comparative Study of the Awareness of Sexual Violence between Male and Female College Student
- Designing Electronic Medical Record using Health Level 7 Development Framework
- Comparative Evaluation of Hormones and Hormone-Like Molecule in Lineage Specification of Human Induced Pluripotent Stem Cells