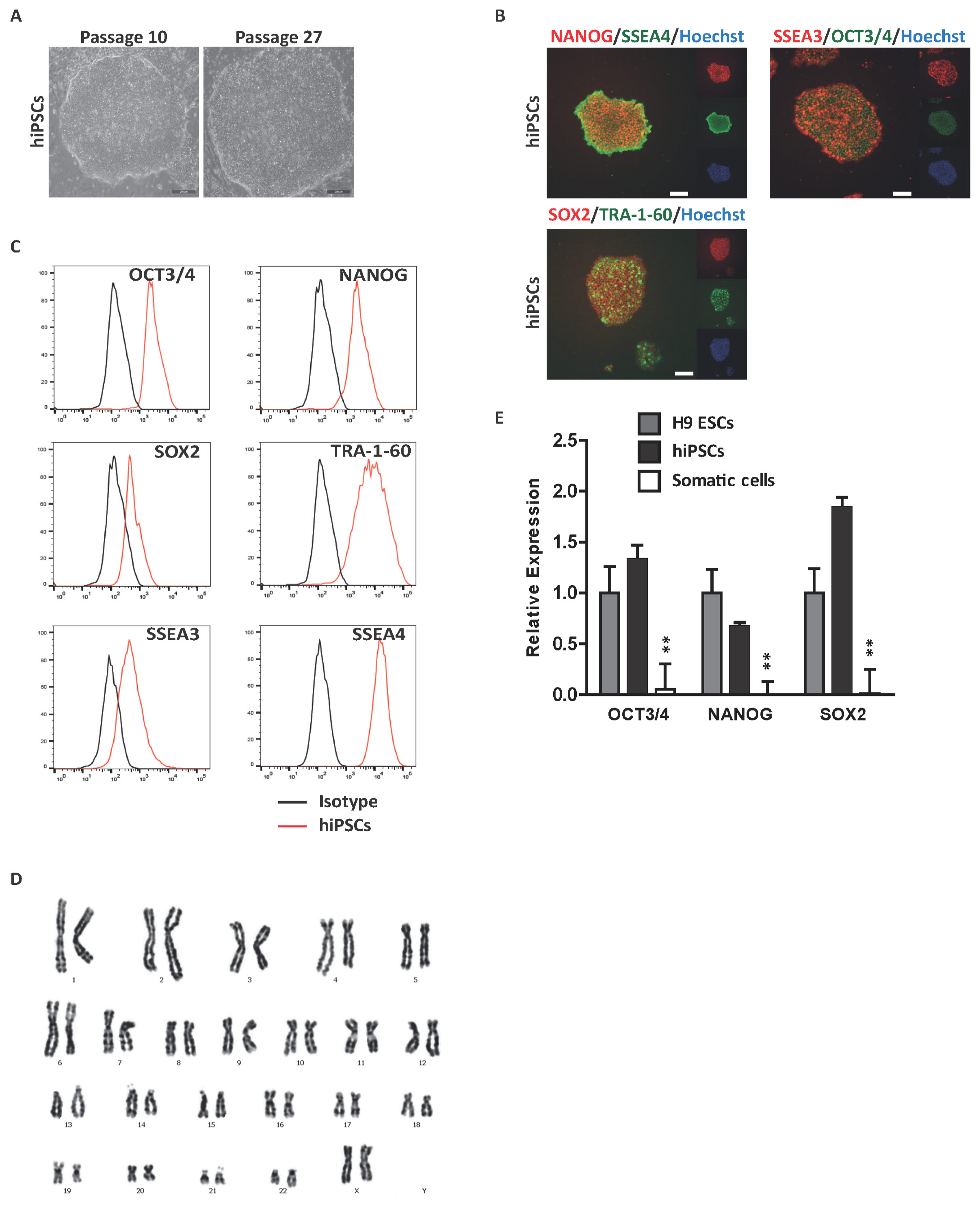
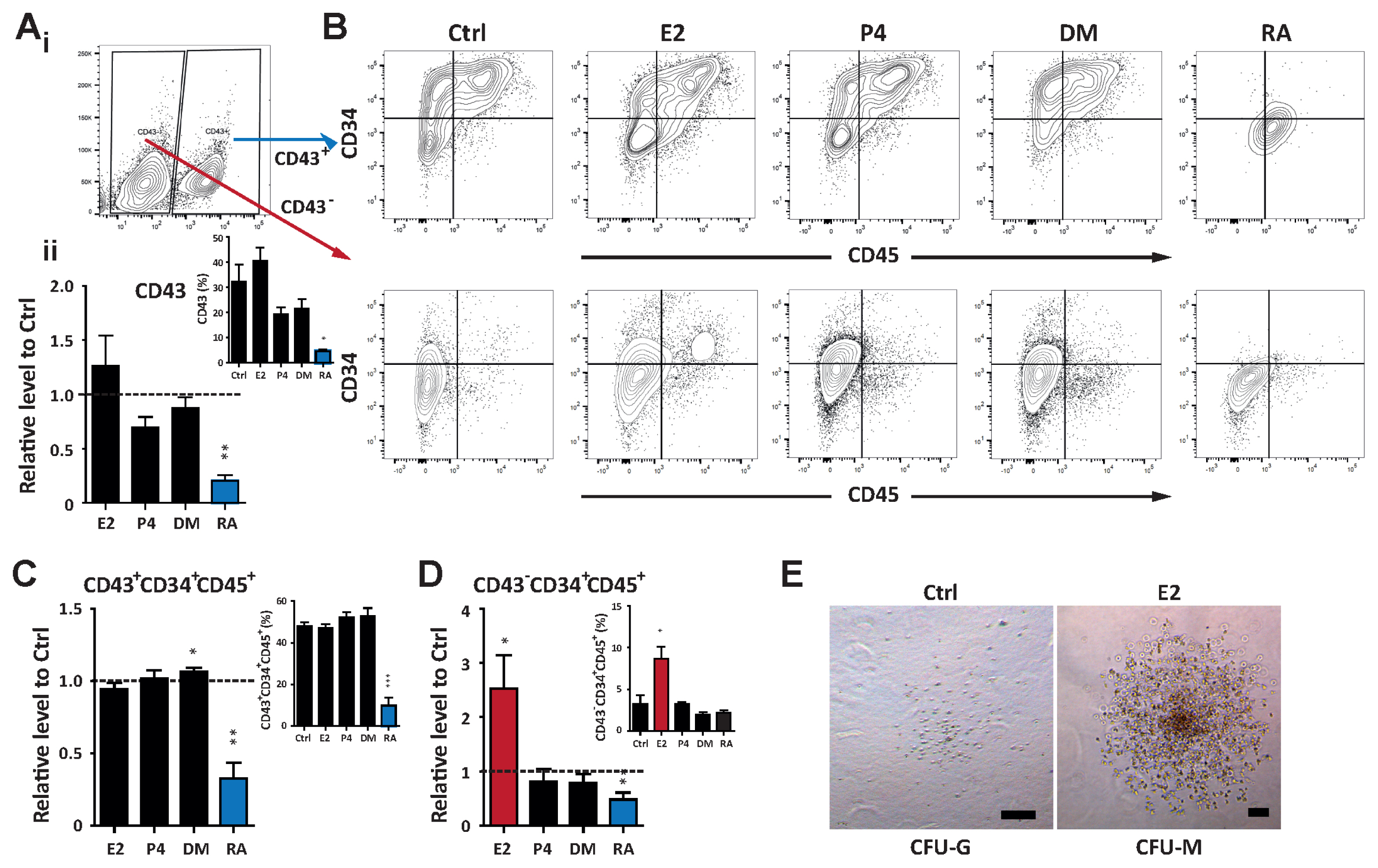
Int J Stem Cells.
2019 Jul;12(2):240-250. 10.15283/ijsc18137.
Comparative Evaluation of Hormones and Hormone-Like Molecule in Lineage Specification of Human Induced Pluripotent Stem Cells
- Affiliations
-
- 1Futuristic Animal Resource & Research Center (FARRC), Korea Research Institute of Bioscience and Biotechnology (KRIBB), Cheongju, Korea. jonglee@kribb.re.kr, sunuk@kribb.re.kr
- 2National Primate Research Center (NPRC), Korea Research Institute of Bioscience and Biotechnology (KRIBB), Cheongju, Korea.
- 3Department of Functional Genomics, KRIBB School of Bioscience, Korea University of Science and Technology (UST), Daejeon, Korea.
- 4Primate Resource Center, Korea Research Institute of Bioscience and Biotechnology, Jeongeup, Korea.
- KMID: 2465895
- DOI: http://doi.org/10.15283/ijsc18137
Abstract
- BACKGROUND AND OBJECTIVES
Proficient differentiation of human pluripotent stem cells (hPSCs) into specific lineages is required for applications in regenerative medicine. A growing amount of evidences had implicated hormones and hormone-like molecules as critical regulators of proliferation and lineage specification during in vivo development. Therefore, a deeper understanding of the hormones and hormone-like molecules involved in cell fate decisions is critical for efficient and controlled differentiation of hPSCs into specific lineages. Thus, we functionally and quantitatively compared the effects of diverse hormones (estradiol 17-β (E2), progesterone (P4), and dexamethasone (DM)) and a hormone-like molecule (retinoic acid (RA)) on the regulation of hematopoietic and neural lineage specification.
METHODS AND RESULTS
We used 10 nM E2, 3 μM P4, 10 nM DM, and 10 nM RA based on their functional in vivo developmental potential. The sex hormone E2 enhanced functional activity of hematopoietic progenitors compared to P4 and DM, whereas RA impaired hematopoietic differentiation. In addition, E2 increased CD34âºCD45⺠cells with progenitor functions, even in the CD43â» population, a well-known hemogenic marker. RA exhibited lineage-biased potential, preferentially committing hPSCs toward the neural lineage while restricting the hematopoietic fate decision.
CONCLUSIONS
Our findings reveal unique cell fate potentials of E2 and RA treatment and provide valuable differentiation information that is essential for hPSC applications.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008; 453:306–313. DOI: 10.1038/nature07038. PMID: 18480811.
Article2. Wahlster L, Daley GQ. Progress towards generation of human haematopoietic stem cells. Nat Cell Biol. 2016; 18:1111–1117. DOI: 10.1038/ncb3419. PMID: 27723718.
Article3. Espin-Palazon R, Weijts B, Mulero V, Traver D. Proinflammatory signals as fuel for the fire of hematopoietic stem cell emergence. Trends Cell Biol. 2018; 28:58–66. DOI: 10.1016/j.tcb.2017.08.003. PMID: 28882414.
Article4. Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014; 505:555–558. DOI: 10.1038/nature12932. PMID: 24451543. PMCID: 4015622.
Article5. Varshney MK, Inzunza J, Lupu D, Ganapathy V, Antonson P, Rüegg J, Nalvarte I, Gustafsson JÅ. Role of estrogen receptor beta in neural differentiation of mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2017; 114:E10428–E10437. DOI: 10.1073/pnas.1714094114. PMID: 29133394. PMCID: 5715781.
Article6. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010; 465:803–807. DOI: 10.1038/nature09091. PMID: 20445538.
Article7. Canciello A, Russo V, Berardinelli P, Bernabò N, Muttini A, Mattioli M, Barboni B. Progesterone prevents epithelial-mesenchymal transition of ovine amniotic epithelial cells and enhances their immunomodulatory properties. Sci Rep. 2017; 7:3761. DOI: 10.1038/s41598-017-03908-1. PMID: 28630448. PMCID: 5476612.
Article8. Nürnberg E, Horschitz S, Schloss P, Meyer-Lindenberg A. Basal glucocorticoid receptor activation induces proliferation and inhibits neuronal differentiation of human induced pluripotent stem cell-derived neuronal precursor cells. J Steroid Biochem Mol Biol. 2018; 182:119–126. DOI: 10.1016/j.jsbmb.2018.04.017. PMID: 29751108.
Article9. Narla A, Dutt S, McAuley JR, Al-Shahrour F, Hurst S, McConkey M, Neuberg D, Ebert BL. Dexamethasone and lenalidomide have distinct functional effects on erythropoiesis. Blood. 2011; 118:2296–2304. DOI: 10.1182/blood-2010-11-318543. PMID: 21527522. PMCID: 3162357.
Article10. Rhinn M, Dollé P. Retinoic acid signalling during development. Development. 2012; 139:843–858. DOI: 10.1242/dev.065938. PMID: 22318625.
Article11. Tagliaferri D, De Angelis MT, Russo NA, Marotta M, Ceccarelli M, Del Vecchio L, De Felice M, Falco G. Retinoic acid specifically enhances embryonic stem cell metastate marked by Zscan4. PLoS One. 2016; 11:e0147683. DOI: 10.1371/journal.pone.0147683. PMID: 26840068. PMCID: 4740454.
Article12. Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007; 8:755–765. DOI: 10.1038/nrn2212. PMID: 17882253.
Article13. Addae C, Yi X, Gernapudi R, Cheng H, Musto A, Martinez-Ceballos E. All-trans-retinoid acid induces the differentiation of encapsulated mouse embryonic stem cells into GABAergic neurons. Differentiation. 2012; 83:233–241. DOI: 10.1016/j.diff.2012.03.001. PMID: 22466603. PMCID: 3334465.
Article14. Gupta S, Sivalingam D, Hain S, Makkar C, Sosa E, Clark A, Butler SJ. Deriving dorsal spinal sensory interneurons from human pluripotent stem cells. Stem Cell Reports. 2018; 10:390–405. DOI: 10.1016/j.stemcr.2017.12.012. PMID: 29337120. PMCID: 5832443.
Article15. Podleśny-Drabiniok A, Sobska J, de Lera AR, Gołembiowska K, Kamińska K, Dollé P, Cebrat M, Krężel W. Distinct retinoic acid receptor (RAR) isotypes control differentiation of embryonal carcinoma cells to dopaminergic or striatopallidal medium spiny neurons. Sci Rep. 2017; 7:13671. DOI: 10.1038/s41598-017-13826-x. PMID: 29057906. PMCID: PMC5651880.
Article16. Cabezas-Wallscheid N, Buettner F, Sommerkamp P, Klimmeck D, Ladel L, Thalheimer FB, Pastor-Flores D, Roma LP, Renders S, Zeisberger P, Przybylla A, Schönberger K, Scognamiglio R, Altamura S, Florian CM, Fawaz M, Vonficht D, Tesio M, Collier P, Pavlinic D, Geiger H, Schroeder T, Benes V, Dick TP, Rieger MA, Stegle O, Trumpp A. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell. 2017; 169:807–823.e19. DOI: 10.1016/j.cell.2017.04.018. PMID: 28479188.
Article17. Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia. 2002; 16:1896–1905. DOI: 10.1038/sj.leu.2402718. PMID: 12357341.
Article18. Rönn RE, Guibentif C, Moraghebi R, Chaves P, Saxena S, Garcia B, Woods NB. Retinoic acid regulates hematopoietic development from human pluripotent stem cells. Stem Cell Reports. 2015; 4:269–281. DOI: 10.1016/j.stemcr.2015.01.009. PMID: 25680478. PMCID: 4325193.
Article19. Lee JH, Lee JB, Shapovalova Z, Fiebig-Comyn A, Mitchell RR, Laronde S, Szabo E, Benoit YD, Bhatia M. Somatic transcriptome priming gates lineage-specific differentiation potential of human-induced pluripotent stem cell states. Nat Commun. 2014; 5:5605. DOI: 10.1038/ncomms6605. PMID: 25465724.
Article20. Lee JH, Salci KR, Reid JC, Orlando L, Tanasijevic B, Shapovalova Z, Bhatia M. Brief report: human acute myeloid leukemia reprogramming to pluripotency is a rare event and selects for patient hematopoietic cells devoid of leukemic mutations. Stem Cells. 2017; 35:2095–2102. DOI: 10.1002/stem.2655. PMID: 28758276.
Article21. Angelos MG, Abrahante JE, Blum RH, Kaufman DS. Single cell resolution of human hematoendothelial cells defines transcriptional signatures of hemogenic endothelium. Stem Cells. 2018; 36:206–217. DOI: 10.1002/stem.2739. PMID: 29139170. PMCID: PMC5914515.
Article22. Vo LT, Kinney MA, Liu X, Zhang Y, Barragan J, Sousa PM, Jha DK, Han A, Cesana M, Shao Z, North TE, Orkin SH, Doulatov S, Xu J, Daley GQ. Regulation of embryonic haematopoietic multipotency by EZH1. Nature. 2018; 553:506–510. DOI: 10.1038/nature25435. PMID: 29342143. PMCID: 5785461.
Article23. Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006; 108:2095–2105. DOI: 10.1182/blood-2006-02-003327. PMID: 16757688. PMCID: 1895535.
Article24. Illing A, Liu P, Ostermay S, Schilling A, de Haan G, Krust A, Amling M, Chambon P, Schinke T, Tuckermann JP. Estradiol increases hematopoietic stem and progenitor cells independent of its actions on bone. Haematologica. 2012; 97:1131–1135. DOI: 10.3324/haematol.2011.052456. PMID: 22371175. PMCID: 3409808.
Article25. Kim HR, Lee JH, Heo HR, Yang SR, Ha KS, Park WS, Han ET, Song H, Hong SH. Improved hematopoietic differentiation of human pluripotent stem cells via estrogen receptor signaling pathway. Cell Biosci. 2016; 6:50. DOI: 10.1186/s13578-016-0111-9. PMID: 27583127. PMCID: 5006567.
Article26. Hong SH, Nah HY, Lee YJ, Lee JW, Park JH, Kim SJ, Lee JB, Yoon HS, Kim CH. Expression of estrogen receptor-alpha and -beta, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Mol Cells. 2004; 18:320–325. PMID: 15650328.27. Baud O, Verney C, Evrard P, Gressens P. Injectable dexamethasone administration enhances cortical GABAergic neuronal differentiation in a novel model of postnatal steroid therapy in mice. Pediatr Res. 2005; 57:149–156. DOI: 10.1203/01.PDR.0000148069.03855.C4. PMID: 15557103.
Article28. Spreer A, Gerber J, Hanssen M, Schindler S, Hermann C, Lange P, Eiffert H, Nau R. Dexamethasone increases hippocampal neuronal apoptosis in a rabbit model of Escherichia coli meningitis. Pediatr Res. 2006; 60:210–215. DOI: 10.1203/01.pdr.0000227553.47378.9f. PMID: 16864706.
Article29. Yu C, Liu Y, Miao Z, Yin M, Lu W, Lv Y, Ding M, Deng H. Retinoic acid enhances the generation of hematopoietic progenitors from human embryonic stem cell-derived hemato-vascular precursors. Blood. 2010; 116:4786–4794. DOI: 10.1182/blood-2010-01-263335. PMID: 20427702.
Article30. Chanda B, Ditadi A, Iscove NN, Keller G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell. 2013; 155:215–227. DOI: 10.1016/j.cell.2013.08.055. PMID: 24074870.
Article31. Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dollé P, Studer M. Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol. 2007; 303:362–375. DOI: 10.1016/j.ydbio.2006.11.021. PMID: 17184764.
Article32. Uehara M, Yashiro K, Mamiya S, Nishino J, Chambon P, Dolle P, Sakai Y. CYP26A1 and CYP26C1 cooperatively regulate anterior-posterior patterning of the developing brain and the production of migratory cranial neural crest cells in the mouse. Dev Biol. 2007; 302:399–411. DOI: 10.1016/j.ydbio.2006.09.045. PMID: 17067568.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem cells: general information and perspectives
- Human Placenta-Derived ECM Supports Tri-Lineage Differentiation of Human Induced Pluripotent Stem Cells
- Lymphoid Lineage γδ T Cells Were Successfully Generated from Human Pluripotent Stem Cells via Hemogenic Endothelium
- Stem Cells in Drug Screening for Neurodegenerative Disease
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy