J Vet Sci.
2019 Jan;20(1):2-9. 10.4142/jvs.2019.20.1.2.
Expression of polo-like kinase 1 in pre-implantation stage murine somatic cell nuclear transfer embryos
- Affiliations
-
- 1Cellular Reprogramming and Embryo Biotechnology Laboratory, Dental Research Institute, BK21 PLUS Dental Life Science, Seoul National University School of Dentistry, Seoul 08826, Korea. sangho@snu.ac.kr
- KMID: 2434771
- DOI: http://doi.org/10.4142/jvs.2019.20.1.2
Abstract
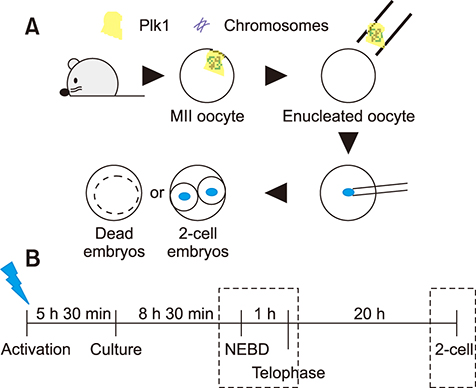
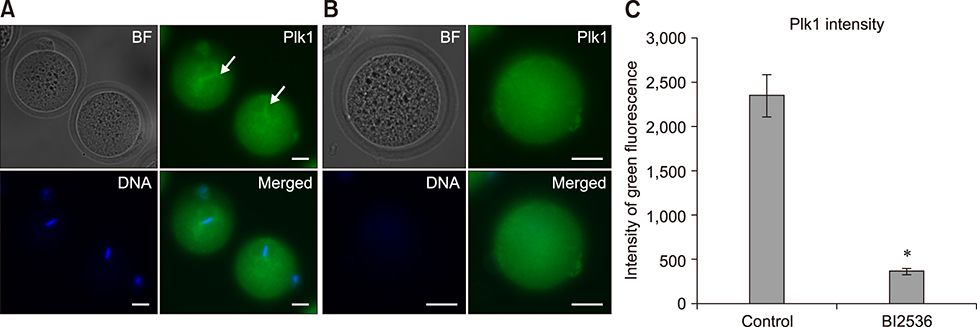
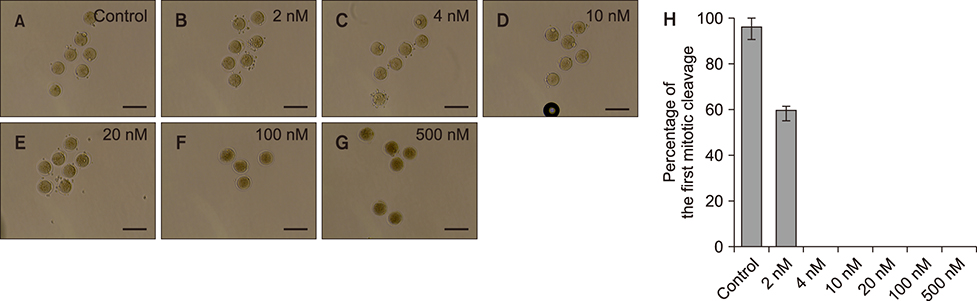
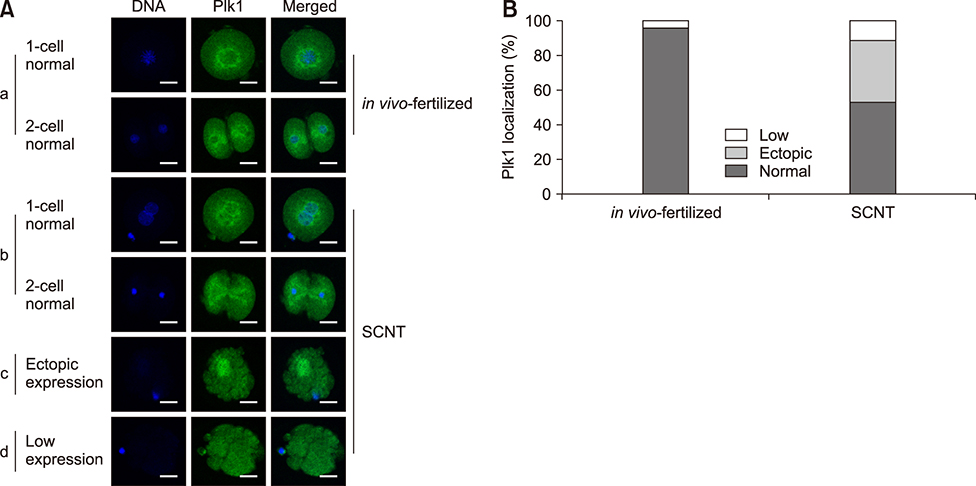
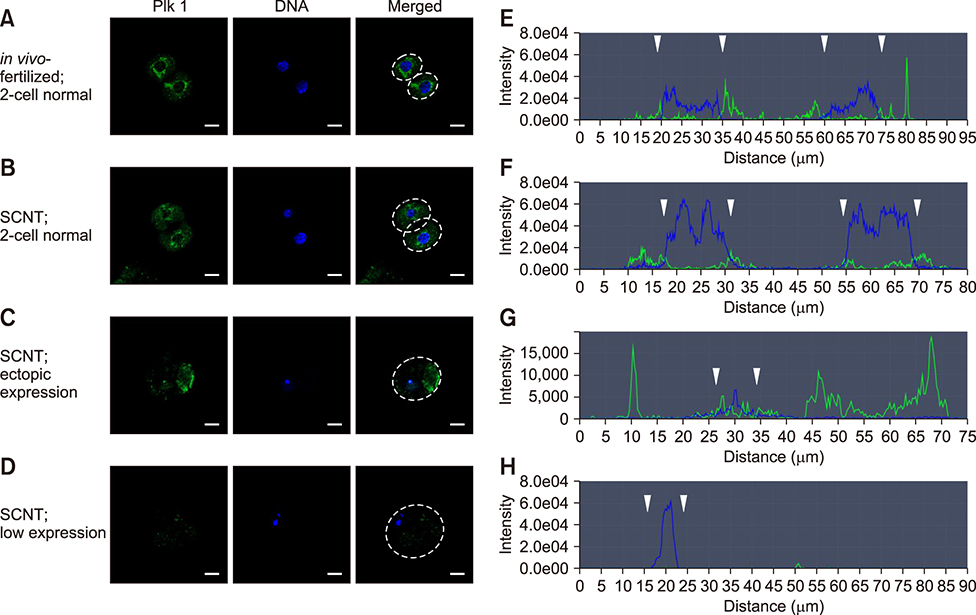
- Somatic cell nuclear transfer (SCNT) has various applications in research, as well as in the medical field and animal husbandry. However, the efficiency of SCNT is low and the accurate mechanism of SCNT in murine embryo development is unreported. In general, the developmental rate of SCNT murine embryos is lower than in vivo counterparts. In previous studies, polo-like kinase 1 (Plk1) was reported to be a crucial element in cell division including centrosome maturation, cytokinesis, and spindle formation. In an initial series of experiments in this study, BI2536, a Plk1 inhibitor, was treated to in vivo-fertilized embryos and the embryos failed to develop beyond the 2-cell stage. This confirmed previous findings that Plk1 is crucial for the first mitotic division of murine embryos. Next, we investigated Plk1's localization and intensity by immunofluorescence analysis. In contrast to normally developed embryos, SCNT murine embryos that failed to develop exhibited two types of Plk1 expressions; a low Plk1 expression pattern and ectopic expression of Plk1. The results show that Plk1 has a critical role in SCNT murine embryos. In conclusion, this study demonstrated that the SCNT murine embryos fail to develop beyond the 2-cell stage, and the embryos show abnormal Plk1 expression patterns, which may one of the main causes of developmental failure of early SCNT murine embryos.
Keyword
MeSH Terms
Figure
Reference
-
1. Baran V, Solc P, Kovarikova V, Rehak P, Sutovsky P. Polo-like kinase 1 is essential for the first mitotic division in the mouse embryo. Mol Reprod Dev. 2013; 80:522–534.
Article2. Bennabi I, Terret ME, Verlhac MH. Meiotic spindle assembly and chromosome segregation in oocytes. J Cell Biol. 2016; 215:611–619.
Article3. Betthauser J, Forsberg E, Augenstein M, Childs L, Eilertsen K, Enos J, Forsythe T, Golueke P, Jurgella G, Koppang R, Lesmeister T, Mallon K, Mell G, Misica P, Pace M, Pfister-Genskow M, Strelchenko N, Voelker G, Watt S, Thompson S, Bishop M. Production of cloned pigs from in vitro systems. Nat Biotechnol. 2000; 18:1055–1059.
Article4. Bucur O, Stancu AL, Muraru MS, Melet A, Petrescu SM, Khosravi-Far R. PLK1 is a binding partner and a negative regulator of FOXO3 tumor suppressor. Discoveries (Craiova). 2014; 2:e16.
Article5. Bui HT, Seo HJ, Park MR, Park JY, Thuan NV, Wakayama T, Kim JH. Histone deacetylase inhibition improves activation of ribosomal RNA genes and embryonic nucleolar reprogramming in cloned mouse embryos. Biol Reprod. 2011; 85:1048–1056.
Article6. Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007; 450:497–502.
Article7. Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996; 380:64–66.
Article8. Chung YG, Eum JH, Lee JE, Shim SH, Sepilian V, Hong SW, Lee Y, Treff NR, Choi YH, Kimbrel EA, Dittman RE, Lanza R, Lee DR. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 2014; 14:777–780.
Article9. Combes G, Alharbi I, Braga LG, Elowe S. Playing polo during mitosis: PLK1 takes the lead. Oncogene. 2017; 36:4819–4827.
Article10. Deuse T, Wang D, Stubbendorff M, Itagaki R, Grabosch A, Greaves LC, Alawi M, Grünewald A, Hu X, Hua X, Velden J, Reichenspurner H, Robbins RC, Jaenisch R, Weissman IL, Schrepfer S. SCNT-derived ESCs with mismatched mitochondria trigger an immune response in allogeneic hosts. Cell Stem Cell. 2015; 16:33–38.
Article11. Hai T, Hao J, Wang L, Jouneau A, Zhou Q. Pluripotency maintenance in mouse somatic cell nuclear transfer embryos and its improvement by treatment with the histone deacetylase inhibitor TSA. Cell Reprogram. 2011; 13:47–56.
Article12. Holtrich U, Wolf G, Bräuninger A, Karn T, Böhme B, Rübsamen-Waigmann H, Strebhardt K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc Natl Acad Sci U S A. 1994; 91:1736–1740.
Article13. Huang J, Zhang H, Yao J, Qin G, Wang F, Wang X, Luo A, Zheng Q, Cao C, Zhao J. BIX-01294 increases pig cloning efficiency by improving epigenetic reprogramming of somatic cell nuclei. Reproduction. 2016; 151:39–49.
Article14. Kang YH, Park JE, Yu LR, Soung NK, Yun SM, Bang JK, Seong YS, Yu H, Garfield S, Veenstra TD, Lee KS. Self-regulated Plk1 recruitment to kinetochores by the Plk1-PBIP1 interaction is critical for proper chromosome segregation. Mol Cell. 2006; 24:409–422.
Article15. Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998; 282:2095–2098.
Article16. Kim J, Ishiguro K, Nambu A, Akiyoshi B, Yokobayashi S, Kagami A, Ishiguro T, Pendas AM, Takeda N, Sakakibara Y, Kitajima TS, Tanno Y, Sakuno T, Watanabe Y. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature. 2015; 517:466–471.
Article17. Kishigami S, Wakayama S, Thuan NV, Ohta H, Mizutani E, Hikichi T, Bui HT, Balbach S, Ogura A, Boiani M, Wakayama T. Production of cloned mice by somatic cell nuclear transfer. Nat Protoc. 2006; 1:125–138.
Article18. Lee KS, Oh DY, Kang YH, Park JE. Self-regulated mechanism of Plk1 localization to kinetochores: lessons from the Plk1-PBIP1 interaction. Cell Div. 2008; 3:4.
Article19. Lénárt P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007; 17:304–315.
Article20. Li Z, Liu J, Li J, Kong Y, Sandusky G, Rao X, Liu Y, Wan J, Liu X. Polo-like kinase 1 (Plk1) overexpression enhances ionizing radiation-induced cancer formation in mice. J Biol Chem. 2017; 292:17461–17472.
Article21. Li Z, Sun X, Chen J, Liu X, Wisely SM, Zhou Q, Renard JP, Leno GH, Engelhardt JF. Cloned ferrets produced by somatic cell nuclear transfer. Dev Biol. 2006; 293:439–448.
Article22. Mallol A, Santaló J, Ibáñez E. Improved development of somatic cell cloned mouse embryos by vitamin C and latrunculin A. PLoS One. 2015; 10:e0120033.
Article23. Matoba S, Liu Y, Lu F, Iwabuchi KA, Shen L, Inoue A, Zhang Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell. 2014; 159:884–895.
Article24. Miyamoto T, Akutsu SN, Fukumitsu A, Morino H, Masatsuna Y, Hosoba K, Kawakami H, Yamamoto T, Shimizu K, Ohashi H, Matsuura S. PLK1-mediated phosphorylation of WDR62/MCPH2 ensures proper mitotic spindle orientation. Hum Mol Genet. 2017; 26:4429–4440.
Article25. Mizutani E, Yamagata K, Ono T, Akagi S, Geshi M, Wakayama T. Abnormal chromosome segregation at early cleavage is a major cause of the full-term developmental failure of mouse clones. Dev Biol. 2012; 364:56–65.
Article26. Niakan KK, Han J, Pedersen RA, Simon C, Pera RA. Human pre-implantation embryo development. Development. 2012; 139:829–841.
Article27. Otsuki J, Nagai Y, Chiba K. Association of spindle midzone particles with polo-like kinase 1 during meiosis in mouse and human oocytes. Reprod Biomed Online. 2009; 18:522–528.
Article28. Raab M, Kappel S, Krämer A, Sanhaji M, Matthess Y, Kurunci-Csacsko E, Calzada-Wack J, Rathkolb B, Rozman J, Adler T, Busch DH, Esposito I, Fuchs H, Gailus-Durner V, Klingenspor M, Wolf E, Sänger N, Prinz F, Angelis MH, Seibler J, Yuan J, Bergmann M, Knecht R, Kreft B, Strebhardt K. Toxicity modelling of Plk1-targeted therapies in genetically engineered mice and cultured primary mammalian cells. Nat Commun. 2011; 2:395.
Article29. Schmucker S, Sumara I. Molecular dynamics of PLK1 during mitosis. Mol Cell Oncol. 2014; 29:e954507.
Article30. Solc P, Kitajima TS, Yoshida S, Brzakova A, Kaido M, Baran V, Mayer A, Samalova P, Motlik J, Ellenberg J. Multiple requirements of PLK1 during mouse oocyte maturation. PLoS One. 2015; 10:e0116783.
Article31. Soung NK, Park JE, Yu LR, Lee KH, Lee JM, Bang JK, Veenstra TD, Rhee K, Lee KS. Plk1-dependent and -independent roles of an ODF2 splice variant, hCenexin1, at the centrosome of somatic cells. Dev Cell. 2009; 16:539–550.
Article32. Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, Gürtler U, Garin-Chesa P, Lieb S, Quant J, Grauert M, Adolf GR, Kraut N, Peters JM, Rettig WJ. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007; 17:316–322.
Article33. Sun SC, Liu HL, Sun QY. Survivin regulates Plk1 localization to kinetochore in mouse oocyte meiosis. Biochem Biophys Res Commun. 2012; 421:797–800.
Article34. Svoboda P. Mammalian zygotic genome activation. Semin Cell Dev Biol. 2018; 84:118–126.
Article35. Tao J, Zhang Y, Zuo X, Hong R, Li H, Liu X, Huang W, Cao Z, Zhang Y. DOT1L inhibitor improves early development of porcine somatic cell nuclear transfer embryos. PLoS One. 2017; 12:e0179436.
Article36. Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle. 2011; 10:1295–1302.
Article37. Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998; 394:369–374.
Article38. Wang YS, He X, Du Y, Su J, Gao M, Ma Y, Hua S, Quan F, Liu J, Zhang Y. Transgenic cattle produced by nuclear transfer of fetal fibroblasts carrying Ipr1 gene at a specific locus. Theriogenology. 2015; 84:608–616.
Article39. Weng Ng WT, Shin JS, Roberts TL, Wang B, Lee CS. Molecular interactions of polo-like kinase 1 in human cancers. J Clin Pathol. 2016; 69:557–562.
Article40. Zhang Z, Chen C, Cui P, Liao Y, Yao L, Zhang Y, Rui R, Ju S. Plk1 inhibition leads to a failure of mitotic division during the first mitotic division in pig embryos. J Assist Reprod Genet. 2017; 34:399–407.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of porcine urine-derived cells as nuclei donor for somatic cell nuclear transfer
- Production of cloned sei whale (Balaenoptera borealis) embryos by interspecies somatic cell nuclear transfer using enucleated pig oocytes
- Cloned calves derived from somatic cell nuclear transfer embryos cultured in chemically defined medium or modified synthetic oviduct fluid
- Mitochondrial and DNA damage in bovine somatic cell nuclear transfer embryos
- Identification of abnormal gene expression in bovine transgenic somatic cell nuclear transfer embryos