J Vet Sci.
2014 Jun;15(2):225-231. 10.4142/jvs.2014.15.2.225.
Identification of abnormal gene expression in bovine transgenic somatic cell nuclear transfer embryos
- Affiliations
-
- 1College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungnam National University, Daejeon 305-764, Korea.
- 2Central Research Center, K-STEMCELL, Seoul 150-101, Korea.
- 3College of Veterinary Medicine, Research Institute of Veterinary Science, Institute of Green Bio Science & Technology, Seoul National University, Seoul 151-742, Korea. bclee@snu.ac.kr
- KMID: 1784643
- DOI: http://doi.org/10.4142/jvs.2014.15.2.225
Abstract
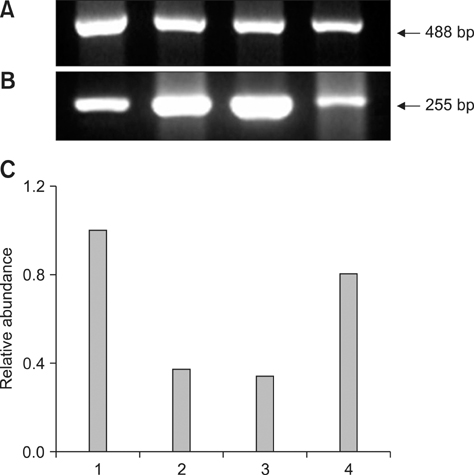
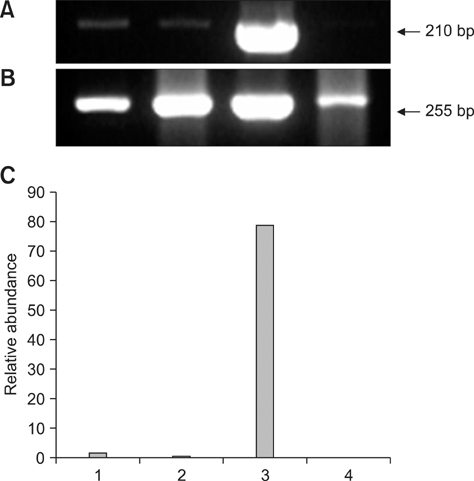
- This study was conducted to investigate the expression of three genes related to early embryonic development in bovine transgenic cloned embryos. To accomplish this, development of bovine transgenic somatic cell nuclear transfer (SCNT) embryos was compared with non-transgenic embryos. Next, mRNA transcription of three specific genes (DNMT1, Hsp 70.1, and Mash2) related to early embryo development in transgenic SCNT embryos was compared between transgenic and non-transgenic SCNTs, parthenogenetic embryos, and in vitro fertilization (IVF) embryos. Transgenic SCNT embryos showed significantly lower rates of development to the blastocyst stage than non-transgenic ones. To investigate normal gene expression, RNA was extracted from ten blastocysts derived from parthenogenesis, IVF, non-transgenic, and transgenic SCNT embryos and reverse-transcribed to synthesize cDNA. The cDNA was then subjected to PCR amplification and semi-quantified. More DNMT1 mRNA was detected in the transgenic SCNT group than the other three groups. Hsp 70.1 mRNA was detected in the IVF embryos, while lower levels were found in SCNT and parthenogenetic embryos. Mash2 mRNA was present at the highest levels in transgenic SCNT embryos. In conclusion, the higher levels of methylation and lower protein synthesis after heat shock in the transgenic SCNT embryos expected based on our results may cause lower embryonic development.
MeSH Terms
-
Animals
Animals, Genetically Modified/genetics
Basic Helix-Loop-Helix Transcription Factors/*genetics/metabolism
Cattle/embryology/*genetics
DNA (Cytosine-5-)-Methyltransferase/*genetics/metabolism
Embryo, Mammalian/embryology/metabolism
Female
Fertilization in Vitro
*Gene Expression Regulation, Developmental
HSP70 Heat-Shock Proteins/*genetics/metabolism
Nuclear Transfer Techniques/veterinary
Parthenogenesis
Pregnancy
RNA, Messenger/genetics/metabolism
Transcription, Genetic
Basic Helix-Loop-Helix Transcription Factors
HSP70 Heat-Shock Proteins
RNA, Messenger
DNA (Cytosine-5-)-Methyltransferase
Figure
Cited by 1 articles
-
Improved preimplantation development of porcine somatic cell nuclear transfer embryos by caffeine treatment
Ghangyong Kim, Pantu Kumar Roy, Xun Fang, Bahia MS Hassan, Jongki Cho
J Vet Sci. 2019;20(3):. doi: 10.4142/jvs.2019.20.e31.
Reference
-
1. Alders M, Hodges M, Hadjantonakis AK, Postmus J, van Wijk I, Bliek J, de Meulemeester M, Westerveld A, Guillemot F, Oudejans C, Little P, Mannens M. The human Achaete-Scute homologue 2 (ASCL2, HASH2) maps to chromosome 11p15.5, close to IGF2 and is expressed in extravillus trophoblasts. Hum Mol Genet. 1997; 6:859–867.
Article2. Arat S, Gibbons J, Rzucidlo SJ, Respess DS, Tumlin M, Stice SL. In vitro development of bovine nuclear transfer embryos from transgenic clonal lines of adult and fetal fibroblast cells of the same genotype. Biol Reprod. 2002; 66:1768–1774.
Article3. Arat S, Rzucidlo SJ, Gibbons J, Miyoshi K, Stice SL. Production of transgenic bovine embryos by transfer of transfected granulosa cells into enucleated oocytes. Mol Reprod Dev. 2001; 60:20–26.
Article4. Bell JC, Smith LC, Rumpf R, Goff AK. Effect of enucleation on protein synthesis during maturation of bovine oocytes in vitro. Reprod Fertil Dev. 1997; 9:603–608.
Article5. Cho J, Bhuiyan MM, Shin S, Park E, Jang G, Kang S, Lee B, Hwang W. Development potential of transgenic somatic cell nuclear transfer embryos according to various factors of donor cell. J Vet Med Sci. 2004; 66:1567–1573.
Article6. Cho JK, Bhuiyan MMU, Jang G, Park ES, Kang SK, Lee BC, Hwang WS. Production of bovine transgenic cloned embryos using prourokinase-transfected somatic cells: effect of expression level of reporter gene. Korean J Embryo Transf. 2002; 17:101–108.7. Cho JK, Lee BC, Park JI, Lim JM, Shin SJ, Kim KY, Lee BD, Hwang WS. Development of bovine oocytes reconstructed with different donor somatic cells with or without serum starvation. Theriogenology. 2002; 57:1819–1828.
Article8. Cho J, Song B, Yong H, Lee D, Koo D, Lee K, Shin S. Differential gene expression in the bovine transgenic nuclear transfer embryos. J Vet Clin. 2007; 24:295–299.9. Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de León FA, Robl JM. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science. 1998; 280:1256–1258.
Article10. Daniels R, Hall V, Trounson AO. Analysis of gene transcription in bovine nuclear transfer embryos reconstructed with granulosa cell nuclei. Biol Reprod. 2000; 63:1034–1040.
Article11. Dean W, Bowden L, Aitchison A, Klose J, Moore T, Meneses JJ, Reik W, Feil R. Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development. 1998; 125:2273–2282.
Article12. Diberardino MA, Hoffner NJ, Matilsky MB. Methods for studying nucleocytoplasmic exchange of nonhistone proteins in embryos. Methods Cell Biol. 1977; 16:141–165.13. Echelard Y, Williams JL, Destrempes MM, Koster JA, Overton SA, Pollock DP, Rapiejko KT, Behboodi E, Masiello NC, Gavin WG, Pommer J, Van Patten SM, Faber DC, Cibelli JB, Meade HM. Production of recombinant albumin by a herd of cloned transgenic cattle. Transgenic Res. 2009; 18:361–376.
Article14. Fukui Y. Effect of follicle cells on the acrosome reaction, fertilization, and developmental competence of bovine oocytes matured in vitro. Mol Reprod Dev. 1990; 26:40–46.
Article15. Guillemot F, Caspary T, Tilghman SM, Copeland NG, Gilbert DJ, Jenkins NA, Anderson DJ, Joyner AL, Rossant J, Nagy A. Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat Genet. 1995; 9:235–242.
Article16. Gurdon JB. Molecular biology in a living cell. Nature. 1974; 248:772–776.
Article17. Gutierrez JA, Guerriero V Jr. Chemical modifications of a recombinant bovine stress-inducible 70 kDa heat-shock protein (Hsp70) mimics Hsp70 isoforms from tissues. Biochem J. 1995; 305:197–203.
Article18. Hirao Y, Naruse K, Kaneda M, Somfai T, Iga K, Shimizu M, Akagi S, Cao F, Kono T, Nagai T, Takenouchi N. Production of fertile offspring from oocytes grown in vitro by nuclear transfer in cattle. Biol Reprod. 2013; 89:57.19. Kanka J, Fulka J Jr, Fulka J, Petr J. Nuclear transplantation in bovine embryo: fine structural and autoradiographic studies. Mol Reprod Dev. 1991; 29:110–116.
Article20. Kanka J, Smith SD, Soloy E, Holm P, Callesen H. Nucleolar ultrastructure in bovine nuclear transfer embryos. Mol Reprod Dev. 1999; 52:253–263.
Article21. Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998; 282:2095–2098.
Article22. Koide T, Ainscough J, Wijgerde M, Surani MA. Comparative analysis of Igf-2/H19 imprinted domain: identification of a highly conserved intergenic DNase I hypersensitive region. Genomics. 1994; 24:1–8.
Article23. Kono T. Nuclear transfer and reprogramming. Rev Reprod. 1997; 2:74–80.
Article24. Lee BC, Yoon KY, Kim JT, Lee KN, Roh SH, Shin TY, Park JY, Kim NR, Joo SC, Baek NY, Lee ES, Lim JM, Lee WK, Hwang WS. Transvaginal ultrasound-guided ovum pick-up (OPU) in cattle. 2. First OPU-IVF derived calves born from pregnant cow in Korea. Korean J Embryo Transf. 1998; 13:77–86.25. Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology. 2000; 53:21–34.
Article26. Razin A, Shemer R. DNA methylation in early development. Hum Mol Genet. 1995; 4:Spec No. 1751–1755.
Article27. Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, Jones PA. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999; 27:2291–2298.
Article28. Sambrook J, Russell DW. Extraction, purification, and analysis of mRNA from eukaryotic cells. Molecular Cloning: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press;2001. p. 7.1–7.88.29. Smith LC. Membrane and intracellular effects of ultraviolet irradiation with Hoechst 33342 on bovine secondary oocytes matured in vitro. J Reprod Fertil. 1993; 99:39–44.
Article30. Smith SD, Soloy E, Kanka J, Holm P, Callesen H. Influence of recipient cytoplasm cell stage on transcription in bovine nucleus transfer embryos. Mol Reprod Dev. 1996; 45:444–450.
Article31. Surani MA, Kothary R, Allen ND, Singh PB, Fundele R, Ferguson-Smith AC, Barton SC. Genome imprinting and development in the mouse. Dev Suppl. 1990; 89–98.
Article32. Tanaka H. Influence of the timing of blastomere isolation after the removal of nocodazole in bovine nuclear transfer. Theriogenology. 1999; 51:1225–1237.
Article33. Weissbach A, Ward C, Bolden A. Eukaryotic DNA methylation and gene expression. Curr Top Cell Regul. 1989; 30:1–21.
Article34. Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod. 1999; 60:996–1005.
Article35. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997; 385:810–813.
Article36. Wrenzycki C, Herrmann D, Carnwath JW, Niemann H. Alterations in the relative abundance of gene transcripts in preimplantation bovine embryos cultured in medium supplemented with either serum or PVA. Mol Reprod Dev. 1999; 53:8–18.
Article37. Wrenzycki C, Wells D, Herrmann D, Miller A, Oliver J, Tervit R, Niemann H. Nuclear transfer protocol affects messenger RNA expression patterns in cloned bovine blastocysts. Biol Reprod. 2001; 65:309–317.
Article38. Yen RW, Vertino PM, Nelkin BD, Yu JJ, El-Deiry W, Cumaraswamy A, Lennon GG, Trask BJ, Celano P, Baylin SB. Isolation and characterization of the cDNA encoding human DNA methyltransferase. Nucleic Acids Res. 1992; 20:2287–2291.
Article39. Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod. 1998; 3:155–163.
Article40. Zakhartchenko V, Mueller S, Alberio R, Schernthaner W, Stojkovic M, Wenigerkind H, Wanke R, Lassnig C, Mueller M, Wolf E, Brem G. Nuclear transfer in cattle with non-transfected and transfected fetal or cloned transgenic fetal and postnatal fibroblasts. Mol Reprod Dev. 2001; 60:362–369.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improved development of somatic cell cloned bovine embryos by a mammary gland epithelia cells in vitro model
- Expression of polo-like kinase 1 in pre-implantation stage murine somatic cell nuclear transfer embryos
- Mitochondrial and DNA damage in bovine somatic cell nuclear transfer embryos
- Microtubule distribution in somatic cell nuclear transfer bovine embryos following control of nuclear remodeling type
- Production of cloned sei whale (Balaenoptera borealis) embryos by interspecies somatic cell nuclear transfer using enucleated pig oocytes