J Vet Sci.
2013 Sep;14(3):235-240. 10.4142/jvs.2013.14.3.235.
Mitochondrial and DNA damage in bovine somatic cell nuclear transfer embryos
- Affiliations
-
- 1College of Veterinary Medicine and Institute of Veterinary Science, Kangwon National University, Chuncheon 200-701, Korea. htcheong@kangwon.ac.kr
- KMID: 1705552
- DOI: http://doi.org/10.4142/jvs.2013.14.3.235
Abstract
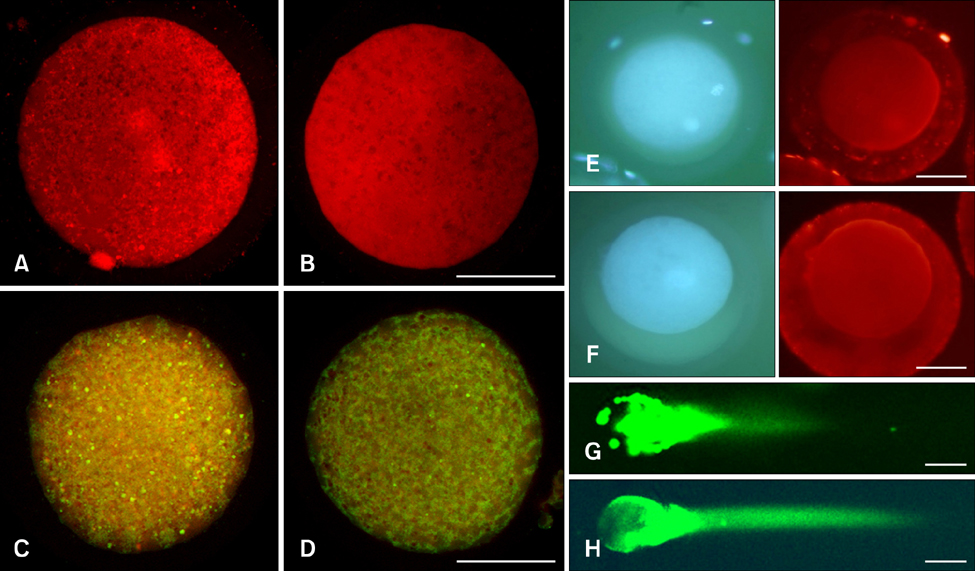
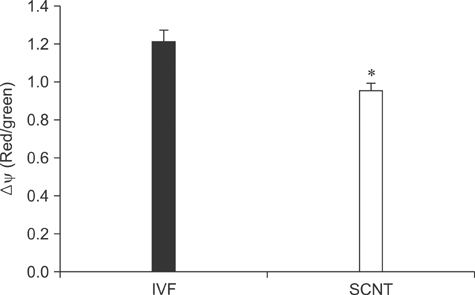
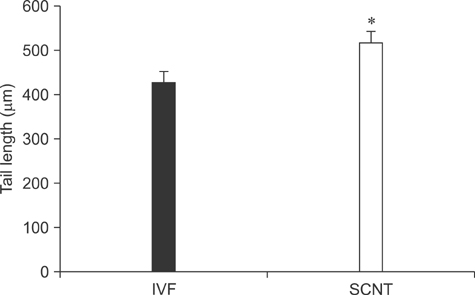
- The generation of reactive oxygen species (ROS) and subsequent mitochondrial and DNA damage in bovine somatic cell nuclear transfer (SCNT) embryos were examined. Bovine enucleated oocytes were electrofused with donor cells and then activated by a combination of Ca-ionophore and 6-dimethylaminopurine culture. The H2O2 and .OH radical levels, mitochondrial morphology and membrane potential (DeltaPsi), and DNA fragmentation of SCNT and in vitro fertilized (IVF) embryos at the zygote stage were analyzed. The H2O2 (35.6 +/- 1.1 pixels/embryo) and .OH radical levels (44.6 +/- 1.2 pixels/embryo) of SCNT embryos were significantly higher than those of IVF embryos (19.2 +/- 1.5 and 23.8 +/- 1.8 pixels/embryo, respectively, p < 0.05). The mitochondria morphology of SCNT embryos was diffused within the cytoplasm. The DeltaPsi of SCNT embryos was significantly lower (p < 0.05) than that of IVF embryos (0.95 +/- 0.04 vs. 1.21 +/- 0.06, red/green). Moreover, the comet tail length of SCNT embryos was longer than that of IVF embryos (515.5 +/- 26.4 microm vs. 425.6 +/- 25.0 microm, p < 0.05). These results indicate that mitochondrial and DNA damage increased in bovine SCNT embryos, which may have been induced by increased ROS levels.
MeSH Terms
-
Animals
*Apoptosis
Caspase 3/metabolism
Cattle
Colorimetry/veterinary
Comet Assay/veterinary
*DNA Damage
DNA, Mitochondrial/*genetics/metabolism
Embryo Transfer/veterinary
Embryo, Mammalian/*cytology/embryology
Fertilization in Vitro/veterinary
In Situ Nick-End Labeling/veterinary
Membrane Potential, Mitochondrial
Microscopy, Confocal/veterinary
Microscopy, Fluorescence/veterinary
Mitochondria/*metabolism
Nuclear Transfer Techniques/*veterinary
Reactive Oxygen Species/*metabolism
Caspase 3
DNA, Mitochondrial
Reactive Oxygen Species
Figure
Reference
-
1. Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989; 41:183–197.
Article2. Albano E, Bellomo G, Parola M, Carini R, Dianzani MU. Stimulation of lipid peroxidation increases the intracellular calcium content of isolated hepatocytes. Biochimica et Biophysica Acta. 1991; 1091:310–316.
Article3. Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod. 1975; 12:260–274.4. Choi JY, Kim CI, Park CK, Yang BK, Cheong HT. Effect of activation time on the nuclear remodeling and in vitro development of nuclear transfer embryos derived from bovine somatic cells. Mol Reprod Dev. 2004; 69:289–295.
Article5. Cox MM, Goodman MF, Kreuzer KN, Sherratt DJ, Sandler SJ, Marians KJ. The importance of repairing stalled replication forks. Nature. 2000; 404:37–41.
Article6. Fahrudin M, Otoi T, Karja NWK, Mori M, Murakami M, Suzuki T. Analysis of DNA fragmentation in bovine somatic nuclear transfer embryos using TUNEL. Reproduction. 2002; 124:813–819.
Article7. Garry FB, Adams R, McCann JP, Odde KG. Postnatal characteristics of calves produced by nuclear transfer cloning. Theriogenology. 1996; 45:141–152.
Article8. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998; 281:1309–1312.
Article9. Halliwell B, Aruoma OI. DNA damage by oxygen-derived species: its mechanism and measurement in mammalian systems. FEBS Lett. 1991; 281:9–19.
Article10. Hao Y, Lai L, Mao J, Im GS, Bonk A, Prather RS. Apoptosis and in vitro development of preimplantation porcine embryos derived in vitro or by nuclear transfer. Biol Reprod. 2003; 69:501–507.
Article11. Hashimoto S, Minami N, Yamada M, Imai H. Excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: relevance to intracellular reactive oxygen species and glutathione contents. Mol Reprod Dev. 2000; 56:520–526.
Article12. Henle ES, Linn S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J Biol Chem. 1997; 272:19095–19098.
Article13. Hwang IS, Bae HK, Park CK, Yang BK, Cheong HT. Generation of reactive oxygen species in bovine somatic cell nuclear transfer embryos during micromanipulation procedures. Reprod Dev Biol. 2012; 36:49–53.14. Inoue K, Kohda T, Lee J, Ogonuki N, Mochida K, Noguchi Y, Tanemura K, Kaneko-Ishino T, Ishino F, Ogura A. Faithful expression of imprinted genes in cloned mice. Science. 2002; 295:297.
Article15. Kang YK, Koo DB, Park JS, Choi YH, Chung AS, Lee KK, Han YM. Aberrant methylation of donor genome in cloned bovine embryos. Nat Genet. 2001; 28:173–177.
Article16. Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effect of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004; 62:1186–1197.
Article17. Kwon DJ, Lee YM, Hwang IS, Park CK, Yang BK, Cheong HT. Microtubule distribution in somatic cell nuclear transfer bovine embryos following control of nuclear remodeling type. J Vet Sci. 2010; 11:93–101.
Article18. Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000; 21:361–370.
Article19. Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000; 25:502–508.
Article20. Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006; 141:357–366.
Article21. Rosenkrans CF Jr, First NL. Effect of free amino acids and vitamins on cleavage and developmental rate of bovine zygotes in vitro. J Anim Sci. 1994; 72:434–437.
Article22. Salgo MG, Stone K, Squadrito GL, Battista JR, Pryor WA. Peroxynitrite causes DNA nicks in plasmid pBR322. Biochem Biophys Res Commun. 1995; 210:1025–1030.
Article23. Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem. 2003; 278:3170–3175.
Article24. Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984; 222:1–15.
Article25. Takahashi M, Saka N, Takahashi H, Kanai Y, Schultz RM, Okano A. Assessment of DNA damage in individual hamster embryos by comet assay. Mol Reprod Dev. 1999; 54:1–7.
Article26. Takahashi M, Nagai T, Hamano S, Kuwayama M, Okamura N, Okano A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol Reprod. 1993; 49:228–232.
Article27. Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000; 118:47–55.
Article28. Thouas GA, Trounson AO, Wolvetang EJ, Jones GM. Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol Reprod. 2004; 71:1936–1942.
Article29. Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998; 13:998–1002.
Article30. Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990; 265:16330–16336.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of abnormal gene expression in bovine transgenic somatic cell nuclear transfer embryos
- Microtubule distribution in somatic cell nuclear transfer bovine embryos following control of nuclear remodeling type
- Cloned calves derived from somatic cell nuclear transfer embryos cultured in chemically defined medium or modified synthetic oviduct fluid
- Production of cloned sei whale (Balaenoptera borealis) embryos by interspecies somatic cell nuclear transfer using enucleated pig oocytes
- Expression of polo-like kinase 1 in pre-implantation stage murine somatic cell nuclear transfer embryos