Korean J Gastroenterol.
2019 Jan;73(1):26-34. 10.4166/kjg.2019.73.1.26.
Effect of 7-day Bismuth Quadruple Therapy versus 14-day Moxifloxacin Triple Therapy for Second-line Helicobacter pylori Eradication Therapy
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Korea. mipark@kosinmed.or.kr
- 2Department of Internal Medicine, Dong-eui Medical Center, Busan, Korea.
- KMID: 2432433
- DOI: http://doi.org/10.4166/kjg.2019.73.1.26
Abstract
- BACKGROUND/AIMS
Both bismuth-containing quadruple therapy and moxifloxacin-containing triple therapy have been suggested as second-line eradication therapy for Helicobacter pylori (H. pylori) infection. We aimed to evaluate the efficacy of 14-day moxifloxacin-containing triple therapy (14-EAM) in second-line H. pylori eradication in comparison to 7-day bismuth-containing quadruple therapy (7-RBMT).
METHODS
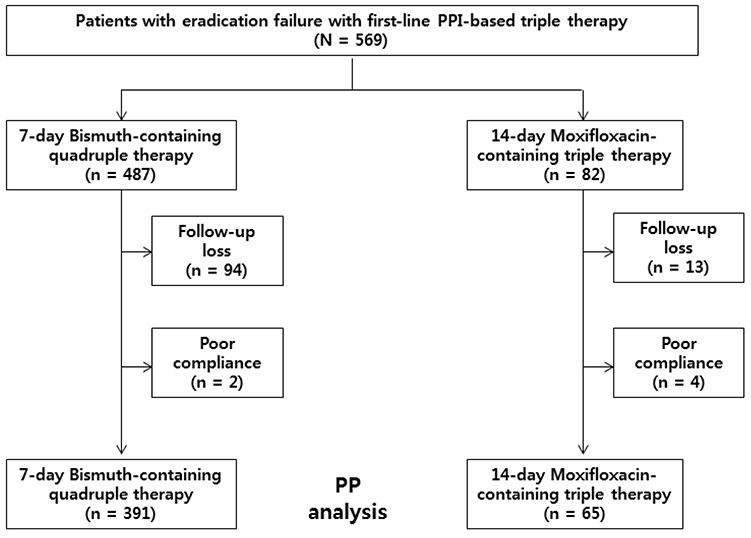
From January 2011 to December 2015, a total of 569 patients who failed to respond to first-line triple therapy and who subsequently received second-line 7-RBMT or 14-EAM were retrospectively enrolled. The eradication rates were identified using per-protocol (PP) analysis. H. pylori eradication was confirmed by a 13C-urea breath test (UBiT-IR300®; Otsuka Electronics, Co., Ltd., Osaka, Japan) or a rapid urease test (CLOtest®; Delta West, Bentley, Australia) at least 4 weeks after completion of eradication therapy.
RESULTS
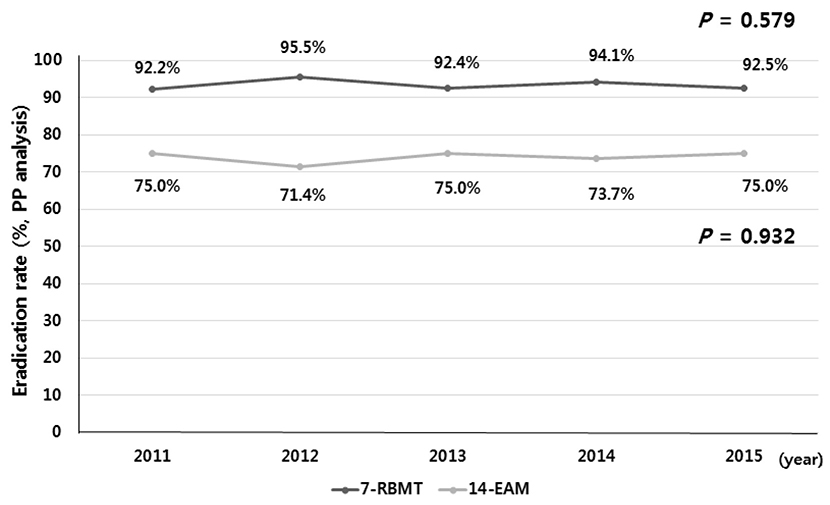
A total of 487 and 82 patients received 7-RBMT and 14-EAM, respectively. PP eradication rates were 93.6% (366/391; 95% CI, 91.0-95.9%) with 7-RBMT and 73.8% (48/65; 95% CI, 63.1-84.6%) with14-EAM (p < 0.001). Therefore, the eradication rates with 7-RBMT were significantly higher than with 14-EAM according to the PP analysis. The adverse event rate was 17.1% (67/391) with 7-RBMT and 7.7% (5/65) with 14-EAM (p=0.065). In terms of risk factors, multivariate analysis revealed that 14-EAM (OR, 5.47; 95% CI, 2.74-10.93) was related to H. pylori eradication failure.
CONCLUSIONS
7-RBMT may be an effective second-line therapy in patients who failed to respond to first-line triple therapy in Korea, where there is a high prevalence of H. pylori infection.
MeSH Terms
Figure
Reference
-
1. Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995; 9:Suppl 2. S33–S39.2. IARC working group on the evaluation of carcinogenic risks to humans: some industrial chemicals. Lyon, 15-22 February 1994. IARC Monogr Eval Carcinog Risks Hum. 1994; 60:1–560.3. Yeo YH, Shiu SI, Ho HJ, et al. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta-analysis. Gut. 2018; 67:20–27.
Article4. Shin WG, Lee SW, Baik GH, et al. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter. 2016; 21:266–278.5. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014; 29:1371–1386.
Article6. Chinese Society of Gastroenterology. Chinese Study Group on Helicobacter pylori. Liu WZ, Xie Y, et al. Fourth Chinese national consensus report on the management of Helicobacter pylori infection. J Dig Dis. 2013; 14:211–221.7. Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013; 132:1272–1276.
Article8. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017; 66:6–30.9. Lee ST, Lee DH, Lim JH, et al. Efficacy of 7-day and 14-day bismuth-containing quadruple therapy and 7-day and 14-day moxifloxacin-based triple therapy as second-line eradication for Helicobacter pylori infection. Gut Liver. 2015; 9:478–485.
Article10. Cao Z, Chen Q, Zhang W, et al. Fourteen-day optimized levofloxacin-based therapy versus classical quadruple therapy for Helicobacter pylori treatment failures: a randomized clinical trial. Scand J Gastroenterol. 2015; 50:1185–1190.
Article11. Kim MS, Kim N, Kim SE, et al. Long-term follow up Helicobacter pylori reinfection rate after second-line treatment: bismuth-containing quadruple therapy versus moxifloxacin-based triple therapy. BMC Gastroenterol. 2013; 13:138.
Article12. Wu C, Chen X, Liu J, Li MY, Zhang ZQ, Wang ZQ. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011; 16:131–138.
Article13. Zagari RM, Romano M, Ojetti V, et al. Guidelines for the management of Helicobacter pylori infection in Italy: the III working group consensus report 2015. Dig Liver Dis. 2015; 47:903–912.
Article14. Hwang JJ, Lee DH, Lee AR, et al. Efficacy of 14-d vs 7-d moxifloxacin-based triple regimens for second-line Helicobacter pylori eradication. World J Gastroenterol. 2015; 21:5568–5574.15. Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004; 53:1374–1384.
Article16. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010; 59:1143–1153.
Article17. Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013; 62:34–42.18. Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010; 8:59–70.19. Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014; 59:1235–1243.
Article20. Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007; 20:280–322.21. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.22. Di Giulio M, Di Campli E, Di Bartolomeo S, et al. In vitro antimicrobial susceptibility of Helicobacter pylori to nine antibiotics currently used in Central Italy. Scand J Gastroenterol. 2016; 51:263–269.23. Gisbert JP, McNicholl AG. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter. 2017; 22:e.12392.24. Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol. 2013; 25:1134–1140.25. Mégraud F, Lamouliatte H. Review article: the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2003; 17:1333–1343.
Article26. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007; 26:343–357.
Article27. Gisbert JP, Pajares JM. Review article: Helicobacter pylori “rescue” regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol Ther. 2002; 16:1047–1057.28. de Boer WA, Driessen WM, Potters VP, Tytgat GN. Randomized study comparing 1 with 2 weeks of quadruple therapy for eradicating Helicobacter pylori. Am J Gastroenterol. 1994; 89:1993–1997.29. Chung JW, Lee JH, Jung HY, et al. Second-line Helicobacter pylori eradication: a randomized comparison of 1-week or 2-week bismuth-containing quadruple therapy. Helicobacter. 2011; 16:289–294.
Article30. Malfertheiner P, Bazzoli F, Delchier JC, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011; 377:905–913.
Article31. Delchier JC, Malfertheiner P, Thieroff-Ekerdt R. Use of a combination formulation of bismuth, metronidazole and tetracycline with omeprazole as a rescue therapy for eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2014; 40:171–177.32. Kim SE, Park MI, Park SJ, et al. Second-line bismuth-containing quadruple therapy for Helicobacter pylori eradication and impact of diabetes. World J Gastroenterol. 2017; 23:1059–1066.
Article33. Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006; 119:217–224.
Article34. Moayyedi P, Chalmers DM, Axon AT. Patient factors that predict failure of omeprazole, clarithromycin, and tinidazole to eradicate Helicobacter pylori. J Gastroenterol. 1997; 32:24–27.35. Kim SE, Park MI, Park SJ, et al. Trends in Helicobacter pylori eradication rates by first-line triple therapy and related factors in eradication therapy. Korean J Intern Med. 2015; 30:801–807.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ten-day Sequential Therapy versus Bismuth Based Quadruple Therapy as Second Line Treatment for Helicobacter pylori Infection
- The Efficacy of Bismuth-containing Quadruple Therapy after Moxifloxacin-based Sequential Therapy Failure in Helicobacter pylori Eradication
- Recent Trends of Helicobacter pylori Eradication Therapy: Focusing on First Line Treatment
- Helicobacter pylori Eradication Therapy in Korea
- Efficacy of 7-Day and 14-Day Bismuth-Containing Quadruple Therapy and 7-Day and 14-Day Moxifloxacin-Based Triple Therapy as Second-Line Eradication for Helicobacter pylori Infection