Korean J Gastroenterol.
2015 Nov;66(5):261-267. 10.4166/kjg.2015.66.5.261.
Ten-day Sequential Therapy versus Bismuth Based Quadruple Therapy as Second Line Treatment for Helicobacter pylori Infection
- Affiliations
-
- 1Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea. dr9696@nate.com
- KMID: 2373354
- DOI: http://doi.org/10.4166/kjg.2015.66.5.261
Abstract
- BACKGROUND/AIMS
Ten-day sequential therapy has been evaluated as the first line therapy for Helicobacter pylori eradication but studies on sequential therapy as a second line therapy is lacking. The aim of this study was to compare the efficacy of 10-day sequential therapy and quadruple therapy as second line treatment for H. pylori eradication after failure of standard triple therapy.
METHODS
Patients who did not respond to standard triple therapy for H. pylori eradication were assigned to either 10-day sequential or bismuth based quadruple therapy as second line treatment from January 2009 to December 2014 at Yeungnam University Medical Center. Post treatment H. pylori status was determined by rapid urease test, giemsa staining, or 13C-urea breath test. Eradication rate and side effects of both therapies were compared.
RESULTS
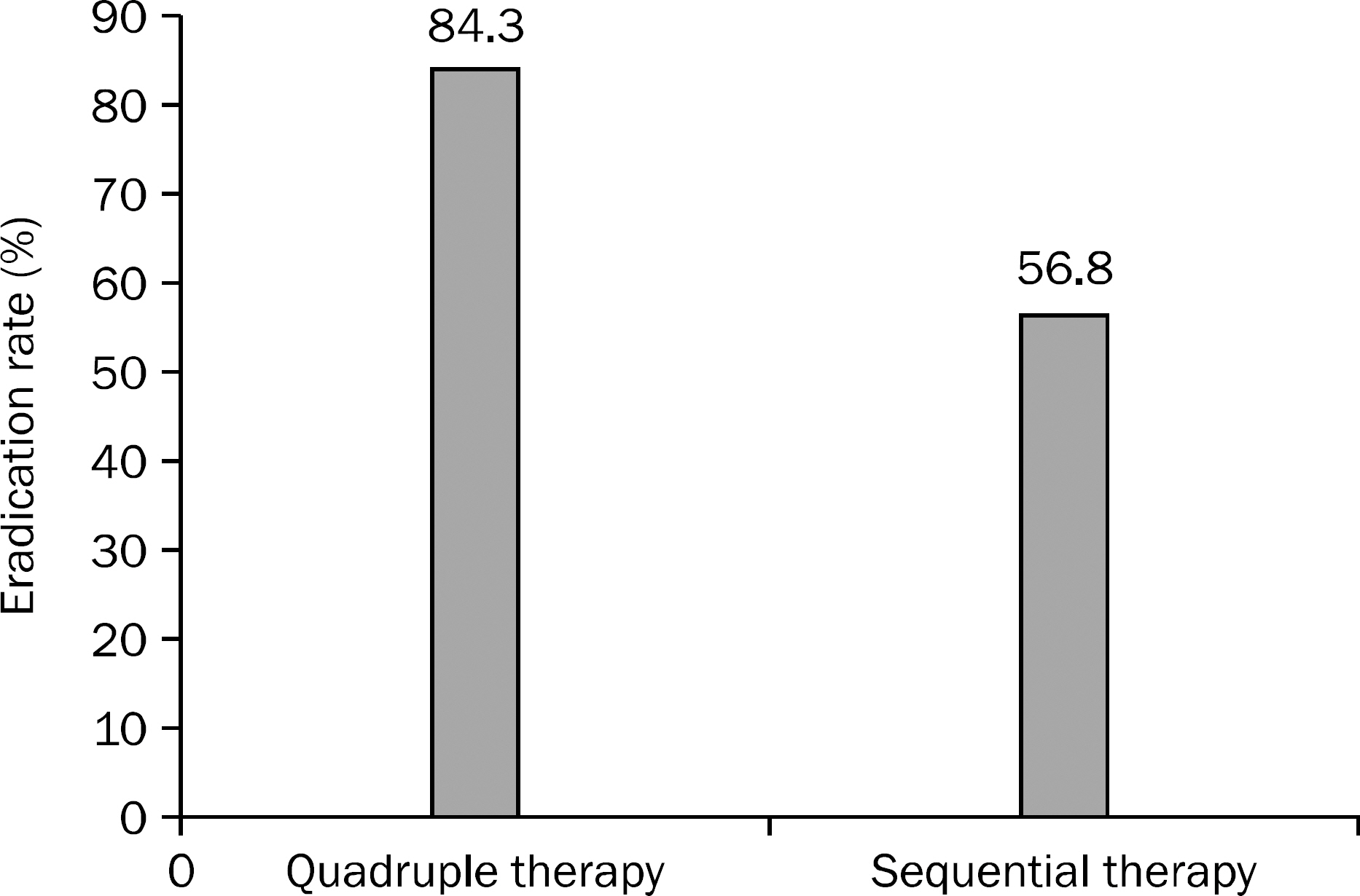
A total of 158 H. pylori infected patients were included and 70 patients were treated by bismuth based quadruple therapy and 88 patients by 10-day sequential therapy. Age and sex were not significantly different between the two groups. Eradication rate was 84.3% (59/70) in quadruple group and 56.8% (50/88) in sequential group. Side effects occurred significantly higher in quadruple group than sequential group (27.1% vs. 11.4%, p=0.011).
CONCLUSIONS
For second line H. pylori eradication after failure of standard triple therapy, bismuth based quadruple therapy showed significantly higher H. pylori eradication rate than 10-day sequential therapy. Further prospective studies are needed to evaluate the efficacy of 10-day sequential therapy as a second line H. pylori eradication treatment.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Anti-Bacterial Agents/adverse effects/pharmacology/*therapeutic use
Bismuth/adverse effects/pharmacology/*therapeutic use
Diarrhea/etiology
Drug Administration Schedule
Drug Therapy, Combination
Female
Helicobacter Infections/*drug therapy
Helicobacter pylori/drug effects
Humans
Male
Middle Aged
Proton Pump Inhibitors/adverse effects/pharmacology/therapeutic use
Retrospective Studies
Risk Factors
Taste Disorders/etiology
Treatment Outcome
Young Adult
Anti-Bacterial Agents
Bismuth
Proton Pump Inhibitors
Figure
Reference
-
References
1. Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.
Article2. Kim SY, Jung SW. Helicobacter pylori eradication therapy in Korea. Korean J Gastroenterol. 2011; 58:67–73.3. Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007; 102:1808–1825.4. Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Conference. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24:1587–1600.5. Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.6. Kim SG, Jung HK, Lee HL, et al. Korean College of Helicobacter and Upper Gastrointestinal Research. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.7. Lee SW, Kim HJ, Kim JG. Treatment of Helicobacter pylori infection in Korea: a systematic review and metaanalysis. J Korean Med Sci. 2015; 30:1001–1009.8. Zullo A, Rinaldi V, Winn S, et al. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000; 14:715–718.9. Kwon JH, Lee DH, Song BJ, et al. Ten-day sequential therapy as first-line treatment for Helicobacter pylori infection in Korea: a retrospective study. Helicobacter. 2010; 15:148–153.10. Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007; 146:556–563.11. Chung JW, Jung YK, Kim YJ, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012; 27:1675–1680.12. Choi HS, Chun HJ, Park SH, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012; 18:2377–2382.13. Choi WH, Park DI, Oh SJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 2008; 51:280–284.14. Kim YS, Kim SJ, Yoon JH, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011; 34:1098–1105.15. Oh HS, Lee DH, Seo JY, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012; 27:504–509.
Article16. Park HG, Jung MK, Jung JT, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment Pharmacol Ther. 2012; 35:56–65.17. Urgesi R, Pelecca G, Cianci R, et al. Helicobacter pylori infection: is sequential therapy superior to standard triple therapy? A single-centre Italian study in treatmentnaive and non-treatmentnaive patients. Can J Gastroenterol. 2011; 25:315–318.18. Ierardi E, Giorgio F, Losurdo G, Di Leo A, Principi M. How antibiotic resistances could change Helicobacter pylori treatment: a matter of geography? World J Gastroenterol. 2013; 19:8168–8180.19. Choi WJ, Do GW, Lee GH. Changes in the antibiotic resistance rates of Helicobacter pylori strains isolated in tertiary medical institutions in Seoul. Korean J Med. 2014; 86:308–313.20. Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. Scand J Gastroenterol. 2001; 36:690–700.21. Yoon JH, Baik GH, Kim YS, et al. Comparison of the eradication rate between 1- and 2-week bismuth-containing quadruple rescue therapies for Helicobacter pylori eradication. Gut Liver. 2012; 6:434–439.22. Lee ST, Lee DH, Lim JH, et al. Efficacy of 7-day and 14-day bismuth-containing quadruple therapy and 7-day and 14-day moxi-floxacin-based triple therapy as second-line eradication for Helicobacter pylori infection. Gut Liver. 2015; 9:478–485.23. Di Caro S, Fini L, Daoud Y, et al. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastroenterol. 2012; 18:5669–5678.24. Cho EJ, Lee DH, Chun JY, et al. Recent trends in the eradication rates of second-line quadruple 14 therapy for Helicobacter pylori and the clinical factors that potentially affect the treatment outcome. Korean J Gastrointest Endosc. 2009; 38:14–19.25. Zagari RM, Romano M, Ojetti V, et al. Guidelines for the management of Helicobacter pylori infection in Italy: The III Working Group Consensus Report 2015. Dig Liver Dis. 2015; 47:903–912.26. Aydin A, Oruc N, Turan I, Ozutemiz O, Tuncyurek M, Musoglu A. The modified sequential treatment regimen containing levofloxacin for Helicobacter pylori eradication in Turkey. Helicobacter. 2009; 14:520–524.27. Liou JM, Chen CC, Chen MJ, et al. Empirical modified sequential therapy containing levofloxacin and high-dose esomeprazole in second-line therapy for Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2011; 66:1847–1852.28. Calhan T, Kahraman R, Sahin A, et al. Efficacy of two levofloxacin-containing second-line therapies for Helicobacter pylori: a pilot study. Helicobacter. 2013; 18:378–383.29. Liou JM, Chen CC, Chen MJ, et al. Taiwan Helicobacter Consortium. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013; 381:205–213.30. De Francesco V, Margiotta M, Zullo A, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006; 144:94–100.31. Hwang JJ, Lee DH, Lee AR, et al. Fourteen- vs seven-day bismuth-based quadruple therapy for second-line Helicobacter pylori eradication. World J Gastroenterol. 2015; 21:8132–8139.32. Kim JY, Kim N, Nam RH, et al. Association of polymorphisms in virulence factor of Helicobacter pylori and gastroduodenal diseases in South Korea. J Gastroenterol Hepatol. 2014; 29:984–991.33. Sugimoto M, Yamaoka Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch Immunol Ther Exp (Warsz). 2009; 57:45–56.34. Chung SJ, Lee DH, Kim N, et al. Comparison of the eradication rates of quadruple therapy between non-ulcer dyspepsia and peptic ulcer disease as a second-line treatment for Helicobacter pylori infection. Korean J Gastrointest Endosc. 2006; 33:63–68.35. Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a metaanalysis. J Clin Pharm Ther. 2009; 34:41–53.36. Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007; 56:1353–1357.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy of Bismuth-containing Quadruple Therapy after Moxifloxacin-based Sequential Therapy Failure in Helicobacter pylori Eradication
- Recent Trends of Helicobacter pylori Eradication Therapy: Focusing on First Line Treatment
- Eradication Rates of Bismuth-based Quadruple Therapy as a Second-line Treatment for Helicobacter pylori Infection
- Current Trends of Helicobacter pylori Eradication in Korea
- Management of Helicobacter pylori Infection in Europe: Focusing on the Maastricht V/Florence Consensus