Korean J Gastroenterol.
2011 Aug;58(2):67-73. 10.4166/kjg.2011.58.2.67.
Helicobacter pylori Eradication Therapy in Korea
- Affiliations
-
- 1Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. sungwoojung@korea.ac.kr
- KMID: 1718412
- DOI: http://doi.org/10.4166/kjg.2011.58.2.67
Abstract
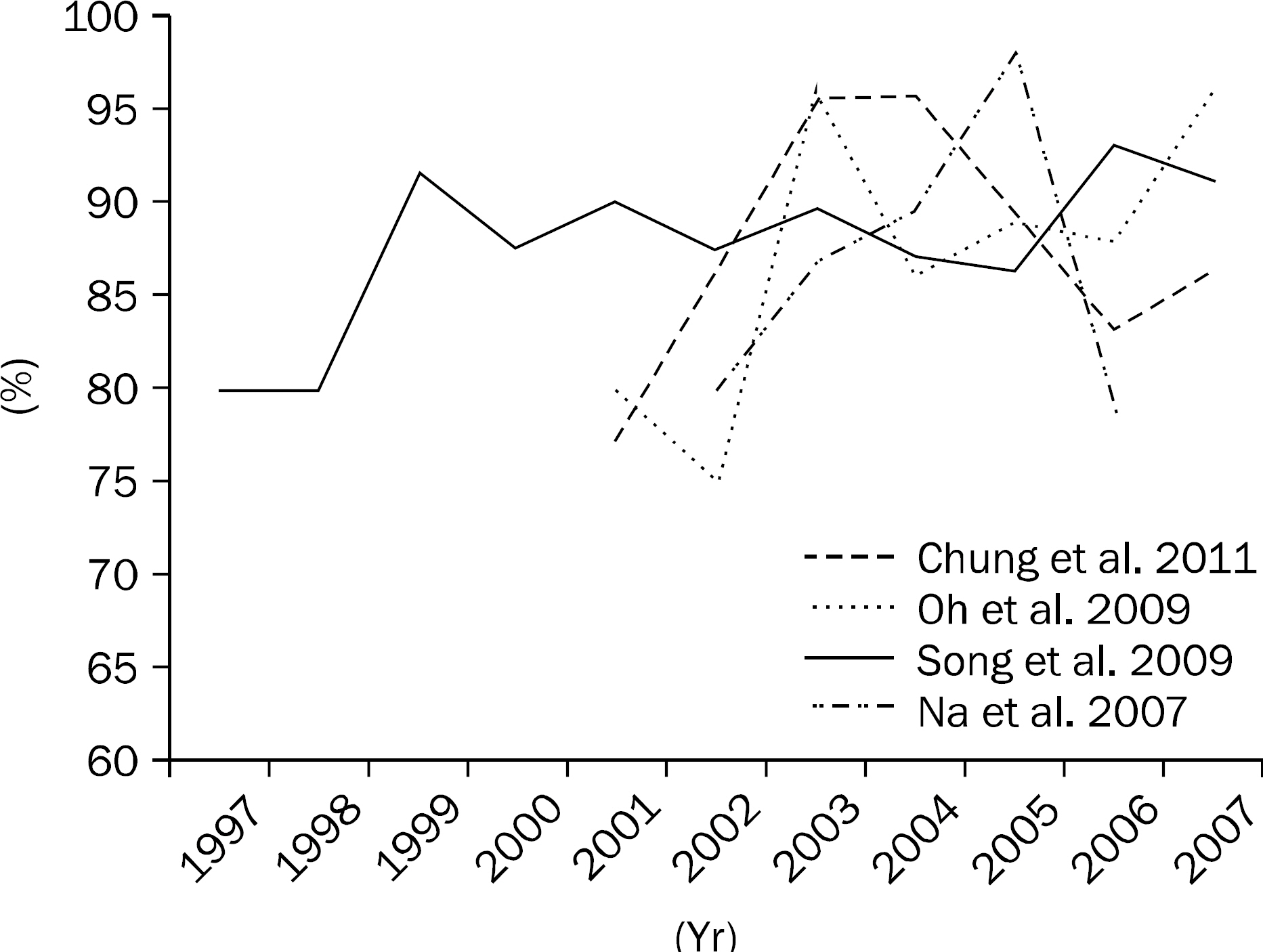
- Helicobacter pylori (H. pylori) is known to be associated with many gastrointestinal diseases including peptic ulcer. In Korea, eradication of H. pylori is recommended for peptic ulcer disease, low grade gastric mucosa-associated lymphoid tissue lymphoma, and early gastric cancer. Standard triple therapy using proton pump inhibitor, clarithromycin, and amoxicillin and bismuth-containing quadruple therapy have been the main first-line and second-line therapy for H. pylori in Korea. Although eradication rate of second-line quadruple therapy remains similar to that of the past, the success rate of eradication with triple therapy has decreased with increasing antimicrobial resistance to H. pylori. There is no standard third-line therapy, and some regimens that incorporate levofloxacin, moxifloxacin, and rifabutin can be used. New regimens such as sequential or concomitant therapy are suggested as alternative treatment for H. pylori. We need more well designed randomized controlled studies to choose proper treatment for H. pylori infection.
Keyword
MeSH Terms
-
Amoxicillin/therapeutic use
Anti-Bacterial Agents/*therapeutic use
Clarithromycin/therapeutic use
Drug Therapy, Combination
Helicobacter Infections/complications/*drug therapy
*Helicobacter pylori
Humans
Lymphoma, B-Cell, Marginal Zone/complications
Peptic Ulcer/complications
Proton Pump Inhibitors/therapeutic use
Republic of Korea
Stomach Neoplasms/complications
Figure
Reference
-
References
1. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002; 347:1175–1186.2. Korean H. pylori Study Group. Diagnosis and treatment of Helicobacter pylori infection in Korea. Korean J Gastroenterol. 1998; 32:275–289.3. Graham DY, Lew GM, Klein PD, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992; 116:705–708.4. Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995; 345:1591–1594.5. Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007; 12:333–340.6. Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010; 15:1–20.7. Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007; 102:1808–1825.8. Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007; 56:772–781.9. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.10. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008; 148:923–931.11. Song JG, Lee SW, Park JY, et al. Trend in the eradication rates of Helicobacter pylori infection in the last 11 years. Korean J Med. 2009; 76:303–310.12. Lee JY, Kim W, Gwak GY, et al. Reinfection rate and clinical manifestation of Helicobacter pylori-positive peptic ulcer disease after triple therapy containing clarithromycin. Korean J Gastroenterol. 2002; 39:93–100.13. Kim BW, Choi MG, Choi H, et al. Pooled analysis of antibiotic therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 1999; 34:42–49.14. Cho DK, Park SY, Kee WJ, et al. The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy. Korean J Gastroenterol. 2010; 55:368–375.15. Choi YS, Cheon JH, Lee JY, et al. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006; 48:156–161.16. Chung JW, Lee GH, Han JH, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011; 58:246–250.17. Chung WC, Lee KM, Paik CN, et al. Inter-departmental differences in the eradication therapy for Helicobacter pylori infection: a single center study. Korean J Gastroenterol. 2009; 53:221–227.18. Na HS, Hong SJ, Yoon HJ, et al. Eradication rate of first-line and second-line therapy for Helicobacter pylori infection, and re-infection rate after successful eradication. Korean J Gastroenterol. 2007; 50:170–175.19. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mu-tation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010; 44:536–543.20. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–4847.21. Kim BG, Lee DH, Ye BD, et al. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: neither treatment duration provides acceptable eradication rate in Korea. Helicobacter. 2007; 12:31–35.
Article22. Jung HS, Shim KN, Park H, et al. Meat-analysis of the H. pylori eradication rates according to the duration of the first-line therapy. Korean J Helicobacter Upper Gastrointest Res. 2008; 8:9–14.23. Gisbert JP, Khorrami S, Calvet X, Pajares JM. Systematic review: Rabeprazole-based therapies in Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003; 17:751–764.24. Laine L. Review article: esomeprazole in the treatment of Helicobacter pylori. Aliment Pharmacol Ther. 2002; 16(Suppl 4):115–118.25. Jung SW, Lee SW, Koo JS, et al. Comparison of proton pump in- hibitor-based triple therapy with generic product of pantoprazole and original pantoprazole for the efficacy for Helicobacter pylori eradication: A randomized study. Helicobacter. 2007; 12:434.26. Kim SY, Lee SW, Jung SW, et al. Comparative study of Helicobacter pylori eradication rates of twice-versus four-times- daily amoxicillin administered with proton pump inhibitor and clarithromycin: a randomized study. Helicobacter. 2008; 13:282–287.27. Kim MN, Kim N, Lee SH, et al. The effects of probiotics on PPI-tri-ple therapy for Helicobacter pylori eradication. Helicobacter. 2008; 13:261–268.28. Song MJ, Park DI, Park JH, et al. The effect of probiotics and mu-coprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter. 2010; 15:206–213.29. Cho EJ, Lee DH, Chun JY, et al. Recent trends in the eradication rates of second-line quadruple therapy for Helicobacter pylori and the clinical factors that potentially affect the treatment outcome. Korean J Gastrointest Endosc. 2009; 38:14–19.30. Oh JH, Kim TH, Cheung DY, et al. Eradication rates of bis-muth-based quadruple therapy as a second-line treatment for Helicobacter pylori infection. Korean J Gastrointest Endosc. 2009; 39:131–135.31. Lee BH, Kim N, Hwang TJ, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010; 15:38–45.32. Jung HS, Shim KN, Baik SJ, et al. Efficacy of levofloxacin-based triple therapy as second-line Helicobacter pylori eradication. Korean J Gastroenterol. 2008; 51:285–290.33. Lee JH, Hong SP, Kwon CI, et al. The efficacy of levofloxacin based triple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2006; 48:19–24.34. Kang JM, Kim N, Lee DH, et al. Second-line treatment for Helicobacter pylori infection: 10-day moxifloxacin-based triple therapy versus 2-week quadruple therapy. Helicobacter. 2007; 12:623–628.35. Lee SK, Lee SW, Park JY, et al. Effectiveness and safety of re-peated quadruple therapy in Helicobacter pylori infection after failure of second-line quadruple therapy. Helicobacter. 2011. in press.36. Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents. 2002; 19:67–70.37. Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007; 56:1353–1357.38. Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009; 104:3069–3079.39. Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a meta-ana-lysis. J Clin Pharm Ther. 2009; 34:41–53.40. Choi WH, Park DI, Oh SJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 2008; 51:280–284.41. Park S, Chun HJ, Kim ES, et al. M1053 The 10-day sequential therapy for Helicobacter pylori eradication in Korea: less effective than expected. Gastroenterology. 2009; 136(5 Suppl 1):A–339. A-340.42. Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009; 14:109–118.43. Fischbach LA, van Zanten S, Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line an-ti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004; 20:1071–1082.44. Kim SY, Lee SW, Kwon BS, et al. Comparative study of Helicobacter pylori eradication rates of 5-day quadruple “concomitant” therapy and 7-day standard triple therapy. Gastroenterology. 2011; 140(5 Suppl 1):S–878.45. Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004; 76:290–301.
Article46. Sugimoto M, Furuta T, Shirai N, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007; 12:317–323.47. Grayson ML, Eliopoulos GM, Ferraro MJ, Moellering RC Jr. Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1989; 8:888–889.48. Shin SK, Lee YC, Youn YH, et al. Comparison of lansoprazole and omeprazole in therapy for Helicobacter pylori infection. Korean J Gastroenterol. 2000; 35:716–723.49. Park GT, Lee SH, Lee HL, et al. Helicobacter pylori eradication by high dose rabeprazole and amoxicillin dual therapy and influence of CYP2C19 genotype on eradication rate. Korean J Helicobacter Res Prac. 2002; 2:192–196.50. Lee DH, Park HJ, Song SY, et al. Evaluation of therapeutic regimens for the treatment of Helicobacter pylori infection. Yonsei Med J. 1996; 37:270–277.51. Shirai N, Sugimoto M, Kodaira C, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007; 63:743–749.52. Furuta T, Shirai N, Takashima M, et al. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001; 69:158–168.53. Furuta T, Shirai N, Takashima M, et al. Effects of genotypic differences in CYP2C19 status on cure rates for Helicobacter pylori infection by dual therapy with rabeprazole plus amoxicillin. Pharmacogenetics. 2001; 11:341–348.54. Bayerdörffer E, Miehlke S, Mannes GA, et al. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology. 1995; 108:1412–1417.55. Kim SY, Jung SW, Kim JH, et al. Effectiveness of three-times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br J Clin Pharmacol. 2011. [Epub ahead of print].56. Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011; 8:79–88.57. Sugimoto M, Furuta T, Shirai N, et al. Treatment strategy to erad-icate Helicobacter pylori infection: impact of pharmacoge-nomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother. 2007; 8:2701–2717.58. Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterol Clin North Am. 2010; 39:465–480.59. Lee JH, Jung HY, Choi KD, Song HJ, Lee GH, Kim JH. The influence of CYP2C19 polymorphism on eradication of Helicobacter pylori: A prospective randomized study of lansoprazole and rabeprazole. Gut Liver. 2010; 4:201–206.60. Kang JM, Kim N, Lee DH, et al. Effec of the CYP2C19 polymorphism on the eradication rate of Helicobacter pylori infection. Korean J Gastroenterol. 2007; 50(5 Suppl):171.61. Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007; 81:521–528.62. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008; 5:321–331.63. Megraud F. Helicobacter pylori and antibiotic resistance. Gut. 2007; 56:1502.64. Gisbert JP. The recurrence of Helicobacter pylori infection: incidence and variables influencing it. A critical review. Am J Gastroenterol. 2005; 100:2083–2099.65. Sugimoto M, Yamaoka Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch Immunol Ther Exp (Warsz). 2009; 57:45–56.66. Mégraud F. H pylori antibiotic resistance: prevalence, im-portance, and advances in testing. Gut. 2004; 53:1374–1384.
Article67. Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy–the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999; 13:1047–1055.
Article68. Qasim A, O'Morain CA, O'Connor HJ. Helicobacter pylori eradication: role of individual therapy constituents and therapy duration. Fundam Clin Pharmacol. 2009; 23:43–52.69. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010; 59:1143–1153.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Approach to Patients after Successful Eradication of Helicobacter pylori
- Eradication Therapy for Helicobacter pylori with Diagnostic Test for Clarithromycin Resistance
- Recent Trends of Helicobacter pylori Eradication Therapy: Focusing on First Line Treatment
- Helicobacter pylori Eradication in Patients Undergoing Gastrectomy: Diagnosis and Therapy
- Indications for Helicobacter pylori Eradication Therapy