Korean J Urol.
2013 Mar;54(3):194-198.
Pyrosequencing Analysis of APC Methylation Level in Human Prostate Tissues: A Molecular Marker for Prostate Cancer
- Affiliations
-
- 1Department of Urology, Chungbuk National University College of Medicine, Cheongju, Korea. urokyj@cbnu.ac.kr
Abstract
- PURPOSE
Epigenetic alterations such as abnormal DNA methylation are associated with many human cancers. Differences in methylation patterns between neoplastic and normal cells can be used to detect cancer. The aim of the present study was to evaluate the effectiveness of detecting Adenomatous polyposis coli (APC) hypermethylation by quantitative pyrosequencing for discriminating between normal and prostate cancer (PCa) cells and for predicting tumor behaviors.
MATERIALS AND METHODS
A total of 218 human prostate tissues obtained from our institute were assessed: 106 specimens of benign prostatic hyperplasia (BPH) and 112 specimens of PCa. The methylation status of APC was analyzed by quantitative pyrosequencing. The association between the APC methylation level and clinicopathological parameters was explored.
RESULTS
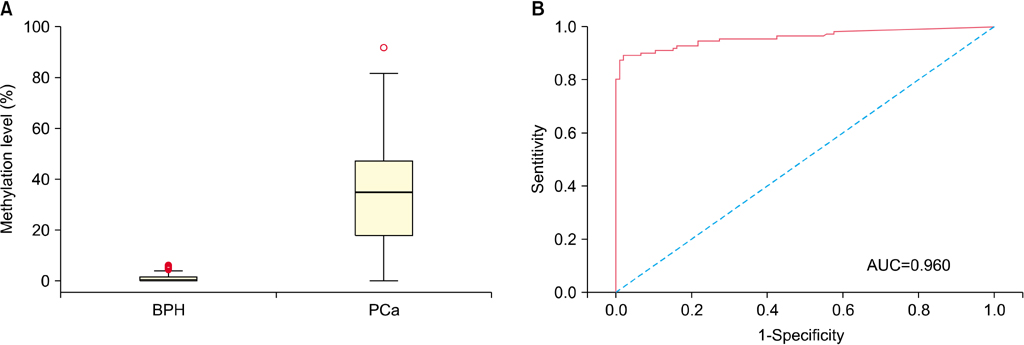
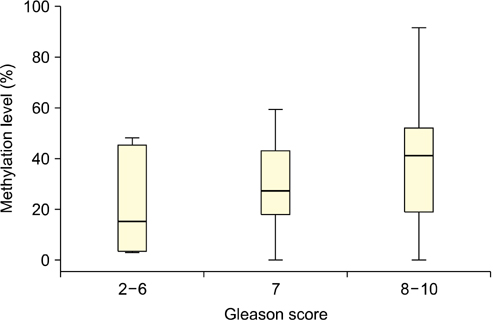
The level of APC methylation was significantly higher in PCa specimens than in BPH specimens (33.3%+/-20.7% vs. 1.3%+/-1.8%, p<0.001). The sensitivity and specificity of APC methylation status in discriminating between PCa and BPH reached 89.3% and 98.1%, respectively. Similar results were obtained after stratification by stage, Gleason score, and prostate-specific antigen level. The APC methylation level correlated positively with Gleason score (p trend=0.016). There was no association between the APC methylation level and the PSA level or staging.
CONCLUSIONS
Our results demonstrate that APC methylation is associated with PCa and its aggressive tumor features.
Keyword
MeSH Terms
Figure
Reference
-
1. van Vlodrop IJ, Niessen HE, Derks S, Baldewijns MM, van Criekinge W, Herman JG, et al. Analysis of promoter CpG island hypermethylation in cancer: location, location, location! Clin Cancer Res. 2011. 17:4225–4231.2. Kim WJ, Kim YJ. Epigenetic biomarkers in urothelial bladder cancer. Expert Rev Mol Diagn. 2009. 9:259–269.3. Hoque MO. DNA methylation changes in prostate cancer: current developments and future clinical implementation. Expert Rev Mol Diagn. 2009. 9:243–257.4. Ushijima T. Detection and interpretation of altered methylation patterns in cancer cells. Nat Rev Cancer. 2005. 5:223–231.5. Kim EJ, Kim YJ, Jeong P, Ha YS, Bae SC, Kim WJ. Methylation of the RUNX3 promoter as a potential prognostic marker for bladder tumor. J Urol. 2008. 180:1141–1145.6. Kim YJ, Yoon HY, Kim SK, Kim YW, Kim EJ, Kim IY, et al. EFEMP1 as a novel DNA methylation marker for prostate cancer: array-based DNA methylation and expression profiling. Clin Cancer Res. 2011. 17:4523–4530.7. Marsh S. Pyrosequencing applications. Methods Mol Biol. 2007. 373:15–24.8. Vasiljevic N, Wu K, Brentnall AR, Kim DC, Thorat MA, Kudahetti SC, et al. Absolute quantitation of DNA methylation of 28 candidate genes in prostate cancer using pyrosequencing. Dis Markers. 2011. 30:151–161.9. Kim JH, Dhanasekaran SM, Prensner JR, Cao X, Robinson D, Kalyana-Sundaram S, et al. Deep sequencing reveals distinct patterns of DNA methylation in prostate cancer. Genome Res. 2011. 21:1028–1041.10. Rai K, Sarkar S, Broadbent TJ, Voas M, Grossmann KF, Nadauld LD, et al. DNA demethylase activity maintains intestinal cells in an undifferentiated state following loss of APC. Cell. 2010. 142:930–942.11. Richiardi L, Fiano V, Vizzini L, De Marco L, Delsedime L, Akre O, et al. Promoter methylation in APC, RUNX3, and GSTP1 and mortality in prostate cancer patients. J Clin Oncol. 2009. 27:3161–3168.12. Henrique R, Ribeiro FR, Fonseca D, Hoque MO, Carvalho AL, Costa VL, et al. High promoter methylation levels of APC predict poor prognosis in sextant biopsies from prostate cancer patients. Clin Cancer Res. 2007. 13:6122–6129.13. Jeronimo C, Henrique R, Hoque MO, Mambo E, Ribeiro FR, Varzim G, et al. A quantitative promoter methylation profile of prostate cancer. Clin Cancer Res. 2004. 10:8472–8478.14. Bastian PJ, Ellinger J, Wellmann A, Wernert N, Heukamp LC, Muller SC, et al. Diagnostic and prognostic information in prostate cancer with the help of a small set of hypermethylated gene loci. Clin Cancer Res. 2005. 11:4097–4106.15. Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, Walsh PC, et al. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004. 64:1975–1986.16. Baden J, Green G, Painter J, Curtin K, Markiewicz J, Jones J, et al. Multicenter evaluation of an investigational prostate cancer methylation assay. J Urol. 2009. 182:1186–1193.17. Gavert N, Ben-Ze'ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007. 102:820–828.