Korean J Physiol Pharmacol.
2018 Nov;22(6):627-636. 10.4196/kjpp.2018.22.6.627.
6-Shogaol reduces progression of experimental endometriosis in vivo and in vitro via regulation of VGEF and inhibition of COX-2 and PGE2-mediated inflammatory responses
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Tongren Hospital of WuHan University (Wuhan Third Hospital), Wuhan 430074, China.
- 2Department of Obstetrics and Gynecology, Hubei Provincial Hospital of Integrated Chinese and Western Medicine, Wuhan 430015, China. JanellAkeribea@yahoo.com
- KMID: 2430085
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.627
Abstract
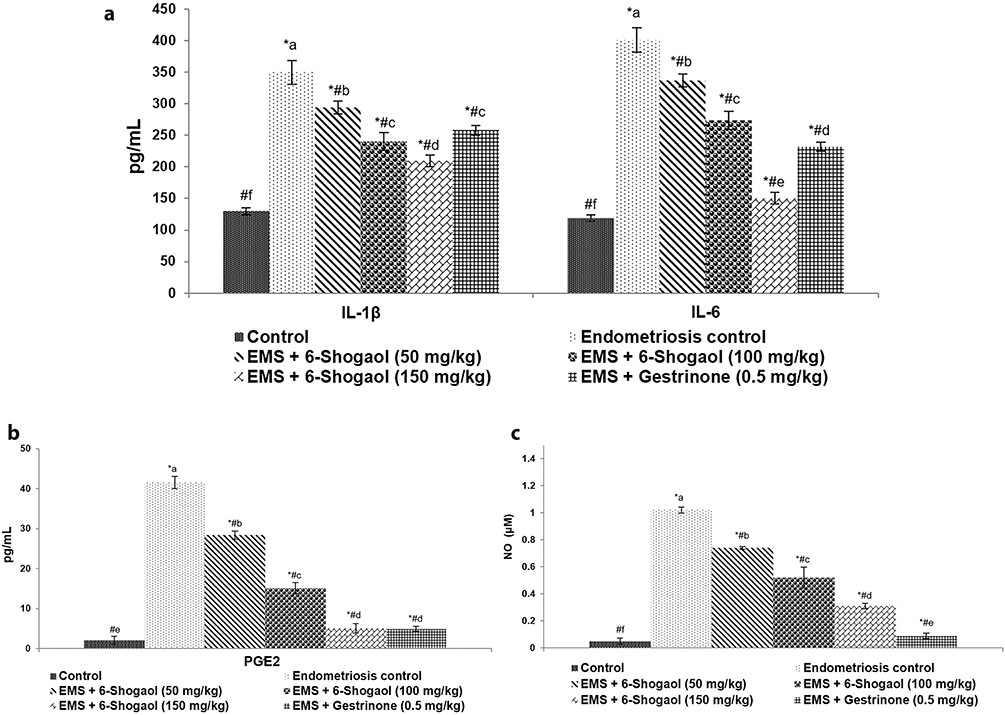
- Endometriosis (EM) is one of the most common gynaecological disorder affecting women in their reproductive age. Mechanisms involved in the pathogenesis of EM remains poorly understood, however inflammatory responses have been reported to be significantly involved. The efficacy of 6-shogaol on proliferation of endometriotic lesions and inflammatory pathways in experimentally-induced EM model was explored in this study. EM was stimulated in Sprague-Dawley rats by implantation of autologous endometrium onto the peritoneum abdominal wall. Separate groups were treated with 6-shogaol (50, 100 or 150 mg/kg b.wt/day) via oral gavage for one month period. Gestrinone (GTN) group received GTN (0.5 mg/kg/day) as positive control. Five weeks after implantation, the spherical volume of ecto-uterine tissues was determined. Treatment with 6-shogaol significantly reduced the implant size. Histological analysis reported atrophy and regression of the lesions. 6-shogaol administration effectively down-regulated NF-κB signaling, VEGF and VEGFR-2 (Flk-1) expression in the endometriotic lesions. Excess production of IL-1β and IL-6 (pro-inflammatory cytokines), PGE2 and nitric oxide (NO) were reduced. Overall, the results of the study reveal the efficacy of 6-shogaol against endometriosis via effectively suppressing proliferation of the lesions and modulating angiogenesis and COX-2/NF-κB-mediated inflammatory cascades.
Keyword
MeSH Terms
-
Abdominal Wall
Atrophy
Dinoprostone
Endometriosis*
Endometrium
Female
Gestrinone
Humans
In Vitro Techniques*
Interleukin-6
Nitric Oxide
Peritoneum
Rats, Sprague-Dawley
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-2
Dinoprostone
Gestrinone
Interleukin-6
Nitric Oxide
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-2
Figure
Reference
-
1. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997; 24:235–258.
Article2. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005; 30:43–52.3. Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E. ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005; 20:2698–2704.
Article4. Ruhland B, Agic A, Krampe J, Diedrich K, Hornung D. Innovations in conservative endometriosis treatment: an updated review. Minerva Ginecol. 2011; 63:247–259.5. Guo SW, Olive DL. Two unsuccessful clinical trials on endometriosis and a few lessons learned. Gynecol Obstet Invest. 2007; 64:24–35.
Article6. Groothuis PG, Nap AW, Winterhager E, Grümmer R. Vascular development in endometriosis. Angiogenesis. 2005; 8:147–156.
Article7. Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci. 2002; 955:89–100. discussion 118, 396-406.
Article8. Bourlev V, Volkov N, Pavlovitch S, Lets N, Larsson A, Olovsson M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction. 2006; 132:501–509.
Article9. Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002; 77:861–870.
Article10. Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann N Y Acad Sci. 2002; 955:183–198. discussion 19-200, 396-406.
Article11. Laschke MW, Elitzsch A, Scheuer C, Vollmar B, Menger MD. Selective cyclo-oxygenase-2 inhibition induces regression of autologous endometrial grafts by down-regulation of vascular endothelial growth factor-mediated angiogenesis and stimulation of caspase-3-dependent apoptosis. Fertil Steril. 2007; 87:163–171.
Article12. Tariverdian N, Theoharides TC, Siedentopf F, Gutiérrez G, Jeschke U, Rabinovich GA, Blois SM, Arck PC. Neuroendocrine-immune disequilibrium and endometriosis: an interdisciplinary approach. Semin Immunopathol. 2007; 29:193–210.
Article13. Wu MH, Shoji Y, Chuang PC, Tsai SJ. Endometriosis: disease pathophysiology and the role of prostaglandins. Expert Rev Mol Med. 2007; 9:1–20.
Article14. Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007; 8:49–62.15. Giudice LC, Kao LC. Endometriosis. Lancet. 2004; 364:1789–1799.
Article16. Chishima F, Hayakawa S, Sugita K, Kinukawa N, Aleemuzzaman S, Nemoto N, Yamamoto T, Honda M. Increased expression of cyclooxygenase-2 in local lesions of endometriosis patients. Am J Reprod Immunol. 2002; 48:50–56.
Article17. Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006; 119:229–240.
Article18. Kim YA, Kim JY, Kim MR, Hwang KJ, Chang DY, Jeon MK. Tumor necrosis factor-alpha-induced cyclooxygenase-2 overexpression in eutopic endometrium of women with endometriosis by stromal cell culture through nuclear factor-kappaB activation. J Reprod Med. 2009; 54:625–630.19. González-Ramos R, Van Langendonckt A, Defrère S, Lousse JC, Colette S, Devoto L, Donnez J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil Steril. 2010; 94:1985–1994.
Article20. Suekawa M, Ishige A, Yuasa K, Sudo K, Aburada M, Hosoya E. Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J Pharmacobiodyn. 1984; 7:836–848.21. Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998; 129:139–144.
Article22. Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, Kadomatsu K, Goto H. Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem Biophys Res Commun. 2007; 362:218–223.
Article23. Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008; 46:409–420.24. Bak MJ, Ok S, Jun M, Jeong WS. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012; 17:8037–8055.
Article25. Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007; 45:683–690.
Article26. Ray A, Vasudevan S, Sengupta S. 6-Shogaol inhibits breast cancer cells and stem cell-like spheroids by modulation of notch signaling pathway and induction of autophagic cell death. PLoS One. 2015; 10:e0137614.
Article27. Seow SLS, Hong SL, Lee GS, Malek SNA, Sabaratnam V. 6-shogaol, a neuroactive compound of ginger (jahe gajah) induced neuritogenic activity via NGF responsive pathways in PC-12 cells. BMC Complement Altern Med. 2017; 17:334.
Article28. Park G, Oh DS, Lee MG, Lee CE, Kim YU. 6-Shogaol, an active compound of ginger, alleviates allergic dermatitis-like skin lesions via cytokine inhibition by activating the Nrf2 pathway. Toxicol Appl Pharmacol. 2016; 310:51–59.
Article29. Han Q, Yuan Q, Meng X, Huo J, Bao Y, Xie G. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget. 2017; 8:42001–42006.30. National Research Council. Committee for the update of the guide for the care and use of laboratory animals. Institute for Laboratory Animal Research. Division on Earth and Life Studies. Guide for the care and use of laboratory animals. 8th ed. Washington, D.C.: National Academy Press;2011.31. Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985; 44:684–694.32. Tang Q, Shang F, Wang X, Yang Y, Chen G, Chen Y, Zhang J, Xu X. Combination use of ferulic acid, ligustrazine and tetrahydropalmatine inhibits the growth of ectopic endometrial tissue: a multi-target therapy for endometriosis rats. J Ethnopharmacol. 2014; 151:1218–1225.
Article33. Yildirim G, Attar R, Ozkan F, Kumbak B, Ficicioglu C, Yesildaglar N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: a preliminary study. Fertil Steril. 2010; 93:1787–1792.
Article34. Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004; 82:Suppl 3. 1008–1013.
Article35. González-Ramos R, Donnez J, Defrère S, Leclercq I, Squifflet J, Lousse JC, Van Langendonckt A. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. 2007; 13:503–509.
Article36. Lousse JC, Van Langendonckt A, González-Ramos R, Defrère S, Renkin E, Donnez J. Increased activation of nuclear factor-kappa B (NF-kappaB) in isolated peritoneal macrophages of patients with endometriosis. Fertil Steril. 2008; 90:217–220.37. Tamura M, Sebastian S, Yang S, Gurates B, Ferrer K, Sasano H, Okamura K, Bulun SE. Up-regulation of cyclooxygenase-2 expression and prostaglandin synthesis in endometrial stromal cells by malignant endometrial epithelial cells. A paracrine effect mediated by prostaglandin E2 and nuclear factor-kappa B. J Biol Chem. 2002; 277:26208–26216.38. Han S, Sidell N. RU486-induced growth inhibition of human endometrial cells involves the nuclear factor-kappa B signaling pathway. J Clin Endocrinol Metab. 2003; 88:713–719.39. Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006; 210:171–186.
Article40. Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR. Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappaB elements. Cell Growth Differ. 1999; 10:353–367.41. Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002; 87:3263–3273.42. Cao WG, Morin M, Metz C, Maheux R, Akoum A. Stimulation of macrophage migration inhibitory factor expression in endometrial stromal cells by interleukin 1, beta involving the nuclear transcription factor NFkappaB. Biol Reprod. 2005; 73:565–570.43. Cao WG, Morin M, Sengers V, Metz C, Roger T, Maheux R, Akoum A. Tumour necrosis factor-alpha up-regulates macrophage migration inhibitory factor expression in endometrial stromal cells via the nuclear transcription factor NF-kappaB. Hum Reprod. 2006; 21:421–428.44. Chen SU, Lee H, Chang DY, Chou CH, Chang CY, Chao KH, Lin CW, Yang YS. Lysophosphatidic acid mediates interleukin-8 expression in human endometrial stromal cells through its receptor and nuclear factor-kappaB-dependent pathway: a possible role in angiogenesis of endometrium and placenta. Endocrinology. 2008; 149:5888–5896.45. Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000; 18:621–663.46. Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001; 2:675–680.
Article47. Boulanger D, Bureau F, Mélotte D, Mainil J, Lekeux P. Increased nuclear factor kappaB activity in milk cells of mastitis-affected cows. J Dairy Sci. 2003; 86:1259–1267.48. Chilov D, Kukk E, Taira S, Jeltsch M, Kaukonen J, Palotie A, Joukov V, Alitalo K. Genomic organization of human and mouse genes for vascular endothelial growth factor C. J Biol Chem. 1997; 272:25176–25183.
Article49. McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 2000; 6:45–55.
Article50. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001; 76:1–10.
Article51. Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, Tsai SJ. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002; 8:1103–1110.
Article52. Wu MY, Chao KH, Yang JH, Lee TH, Yang YS, Ho HN. Nitric oxide synthesis is increased in the endometrial tissue of women with endometriosis. Hum Reprod. 2003; 18:2668–2671.
Article53. Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-kappaB-dependent: studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J Cell Sci. 2003; 116:665–674.54. Lin YJ, Lai MD, Lei HY, Wing LY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006; 147:1278–1286.
Article55. Becker CM, D'Amato RJ. Angiogenesis and antiangiogenic therapy in endometriosis. Microvasc Res. 2007; 74:121–130.
Article56. Machado DE, Berardo PT, Palmero CY, Nasciutti LE. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J Exp Clin Cancer Res. 2010; 29:4.
Article57. Wang HB, Lang JH, Leng JH, Zhu L, Liu ZF, Sun DW. Expression of vascular endothelial growth factor receptors in the ectopic and eutopic endometrium of women with endometriosis. Zhonghua Yi Xue Za Zhi. 2005; 85:1555–1559.58. Celik O, Hascalik S, Elter K, Tagluk ME, Gurates B, Aydin NE. Combating endometriosis by blocking proteasome and nuclear factor-kappaB pathways. Hum Reprod. 2008; 23:2458–2465.59. Ebert AD, Bartley J, David M. Aromatase inhibitors and cyclooxygenase-2 (COX-2) inhibitors in endometriosis: new questions--old answers? Eur J Obstet Gynecol Reprod Biol. 2005; 122:144–150.
Article60. Olivares C, Bilotas M, Buquet R, Borghi M, Sueldo C, Tesone M, Meresman G. Effects of a selective cyclooxygenase-2 inhibitor on endometrial epithelial cells from patients with endometriosis. Hum Reprod. 2008; 23:2701–2708.
Article61. Machado DE, Berardo PT, Landgraf RG, Fernandes PD, Palmero C, Alves LM, Abrao MS, Nasciutti LE. A selective cyclooxygenase-2 inhibitor suppresses the growth of endometriosis with an antiangiogenic effect in a rat model. Fertil Steril. 2010; 93:2674–2679.
Article62. Machado DE, Rodrigues-Baptista KC, Alessandra-Perini J, Soares de Moura R, Santos TA, Pereira KG, Marinho da Silva Y, Souza PJ, Nasciutti LE, Perini JA. Euterpe oleracea extract (Açaí) is a promising novel pharmacological therapeutic treatment for experimental endometriosis. PLoS One. 2016; 11:e0166059.
Article63. Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997; 99:2851–2857.
Article64. Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, Simsa P, Kyama C, Cornillie FJ, Bergqvist A, Fried G, D'Hooghe TM. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod. 2006; 21:1856–1862.
Article65. Mihalyi A, Simsa P, Mutinda KC, Meuleman C, Mwenda JM, D'Hooghe TM. Emerging drugs in endometriosis. Expert Opin Emerg Drugs. 2006; 11:503–524.
Article66. Ergenoğlu AM, Yeniel AÖ, Erbaş O, Aktuğ H, Yildirim N, Ulukuş M, Taskiran D. Regression of endometrial implants by resveratrol in an experimentally induced endometriosis model in rats. Reprod Sci. 2013; 20:1230–1236.
Article67. Zhang Y, Cao H, Hu YY, Wang H, Zhang CJ. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int J Mol Med. 2011; 27:87–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 6-Shogaol and 10-Shogaol Synergize Curcumin in Ameliorating Proinflammatory Mediators via the Modulation of TLR4/TRAF6/ MAPK and NFκB Translocation
- Triclosan Inhibition of Prostaglandin E2 Production in Human Gingival Fibroblast
- Changes in the Expression Pattern of Cyclooxygenase-2, Mapkinases and Related Apoptotic Markers by Different Levels of Estrogen Supplementation in Mature or Ovariectomized Female Rat Heart
- Pro-inflammatory cytokine-induced nuclear factor-kappa B activity in prelabor and in-labor myometrial cells at term gestation
- Suppression of Heme Oxygenase-1 by Prostaglandin E2-Protein Kinase A-A-Kinase Anchoring Protein Signaling Is Central for Augmented Cyclooxygenase-2 Expression in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages