Ann Surg Treat Res.
2019 Jan;96(1):19-26. 10.4174/astr.2019.96.1.19.
Clinical significance of revised microscopic positive resection margin status in ductal adenocarcinoma of pancreatic head
- Affiliations
-
- 1Department of Surgery, Konkuk University Choongju Hospital, Konkuk University School of Medicine, Chungju, Korea.
- 2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dw7722.choi@samsung.com
- 3Department of Surgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 4Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 6Department of Hepatobiliary and Pancreatic Surgery, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2429750
- DOI: http://doi.org/10.4174/astr.2019.96.1.19
Abstract
- PURPOSE
Recent studies have suggested microscopic positive resection margin should be revised according to the presence of tumor cells within 1mm of the margin surface in resected specimens of pancreatic cancer. However, the clinical meaning of this revised margin status for R1 resection margin was not fully clarified.
METHODS
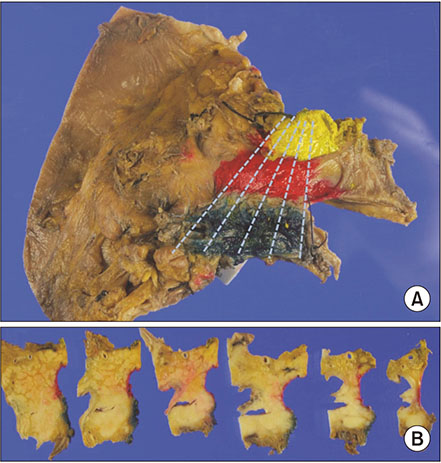
From July 2012 to December 2014, the medical records of 194 consecutive patients who underwent pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head were analyzed retrospectively. They were divided into 3 groups on margin status; revised microscopic negative margin (rR0) - tumor exists more than 1 mm from surgical margin, revised microscopic positive margin (rR1) - tumor present within less than 1 mm from surgical margin, classic microscopic positive margin (cR1) - tumor is exposed to surgical margin.
RESULTS
There were 76 rR0 (39.2%), 100 rR1 (51.5%), and 18 cR1 (9.3%). There was significant difference in disease-free survival rates between cR1 vs. rR1 (8.4 months vs. 24.0 months, P = 0.013). Margin status correlated with local recurrence rate (17.1% in rR0, 26.0% in rR1, and 44.4% in cR1, P = 0.048). There is significant difference in recurrence at tumor bed (11.8% in rR0 vs. 23.0 in rR1, P = 0.050). Of rR1, adjuvant treatment was found to be an independent risk factor for local recurrence (hazard ratio, 0.297; 95% confidence interval, 0.127-0.693, P = 0.005).
CONCLUSION
Revised R1 resection margin (rR1) affects recurrence at the tumor bed. Adjuvant treatment significantly reduced local recurrence of rR1. Accordingly, adjuvant chemoradiation for rR1 group should be taken into account.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinical outcomes of pancreaticoduodenectomy for pancreatic ductal adenocarcinoma depending on preservation or resection of pylorus
Yeon Jin Kim, Sang Hyun Shin, In Woong Han, Youngju Ryu, Naru Kim, Dong Wook Choi, Jin Seok Heo
Ann Hepatobiliary Pancreat Surg. 2020;24(3):269-276. doi: 10.14701/ahbps.2020.24.3.269.
Reference
-
1. Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008; 143:75–83.2. Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995; 221:721–731.
Article3. Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg. 1996; 83:625–631.
Article4. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000; 4:567–579.
Article5. Bassi C, Stocken DD, Olah A, Friess H, Buckels J, Hickey H, et al. Influence of surgical resection and post-operative complications on survival following adjuvant treatment for pancreatic cancer in the ESPAC-1 randomized controlled trial. Dig Surg. 2005; 22:353–363.
Article6. Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006; 10:1199–1210.
Article7. Campbell F, Smith RA, Whelan P, Sutton R, Raraty M, Neoptolemos JP, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology. 2009; 55:277–283.8. Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010; 304:1073–1081.9. Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011; 254:311–319.10. Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a "true" R0 resection? Ann Surg. 2013; 257:731–736.11. Sugiura T, Uesaka K, Mihara K, Sasaki K, Kanemoto H, Mizuno T, et al. Margin status, recurrence pattern, and prognosis after resection of pancreatic cancer. Surgery. 2013; 154:1078–1086.
Article12. Gebauer F, Tachezy M, Vashist YK, Marx AH, Yekebas E, Izbicki JR, et al. Resection margin clearance in pancreatic cancer after implementation of the Leeds Pathology Protocol (LEEPP): clinically relevant or just academic? World J Surg. 2015; 39:493–499.
Article13. Merkow RP, Bilimoria KY, Bentrem DJ, Pitt HA, Winchester DP, Posner MC, et al. National assessment of margin status as a quality indicator after pancreatic cancer surgery. Ann Surg Oncol. 2014; 21:1067–1074.
Article14. Chandrasegaram MD, Goldstein D, Simes J, Gebski V, Kench JG, Gill AJ, et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg. 2015; 102:1459–1472.
Article15. The Royal College of Pathologists. Standards and datasets for reporting cancers. Minimum dataset for the histopathological reporting of pancreatic, ampulla of vater and bile duct carcinoma. London: Royal College of Pathologists;2002.16. Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009; 27:2855–2862.
Article17. Jamieson NB, Foulis AK, Oien KA, Going JJ, Glen P, Dickson EJ, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010; 251:1003–1010.
Article18. Jamieson NB, Chan NI, Foulis AK, Dickson EJ, McKay CJ, Carter CR. The prognostic influence of resection margin clearance following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013; 17:511–521.
Article19. Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg. 2017; 265:565–573.
Article20. Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008; 15:1651–1660.
Article21. Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006; 93:1232–1237.
Article22. Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB (Oxford). 2009; 11:18–24.
Article23. Sobin LH, Gaspodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7th ed. New York: Wiley & Sons;2009.24. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014; 155:977–988.
Article25. Delpero JR, Bachellier P, Regenet N, Le Treut YP, Paye F, Carrere N, et al. Pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a French multicentre prospective evaluation of resection margins in 150 evaluable specimens. HPB (Oxford). 2014; 16:20–33.
Article26. Verbeke CS, Knapp J, Gladhaug IP. Tumour growth is more dispersed in pancreatic head cancers than in rectal cancer: implications for resection margin assessment. Histopathology. 2011; 59:1111–1121.
Article27. Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006; 10:511–518.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant and Adjuvant Treatments for Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma: The Current Status of Pancreatic Ductal Adenocarcinoma Treatment in Japan
- Vein resection in patients with adenocarcinoma of the head of pancreas adherent to the portomesenteric venous axis is beneficial despite a high rate of R1 resection
- Pathological Classification of Panaeatic Cancer and Precancerous Casion
- Clinical Outcome of Positive Margin of Postgastrectomy with Adenocarcinoma of Stomach
- Interpretation of Pathologic Margin after Endoscopic Resection of Gastrointestinal Stromal Tumor