Ann Dermatol.
2018 Dec;30(6):694-700. 10.5021/ad.2018.30.6.694.
The Effects of the 3-OH Group of Kaempferol on Interfollicular Epidermal Stem Cell Fate
- Affiliations
-
- 1Department of Dermatology, Seoul National University Bundang Hospital, Seongnam, Korea. gcpark@snu.ac.kr
- KMID: 2428925
- DOI: http://doi.org/10.5021/ad.2018.30.6.694
Abstract
- BACKGROUND
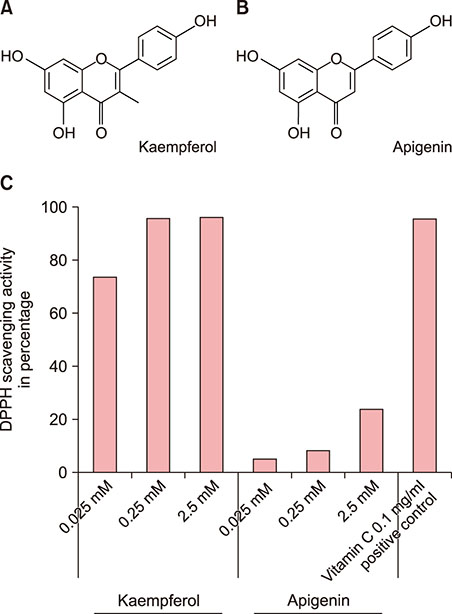
Kaempferol (3,4"²,5,7-tetrahydroxyflavone) is a flavonoid known to have a wide range of pharmacological activities. The 3-OH group in flavonoids has been reported to determine antioxidant activities.
OBJECTIVE
We tested whether kaempferol can affect the expression of integrins and the stem cell fate of interfollicular epidermal stem cells.
METHODS
Skin equivalent (SE) models were constructed, and the expression levels of stem cell markers and basement membrane-related antigens were tested. The immunohistochemical staining patterns of integrins, p63, and proliferating cell nuclear antigen (PCNA) were compared between kaempferol- and apigenin-treated SE models. Reverse transcription-polymerase chain reaction (RT-PCR) was used to evaluate the mRNA expression of integrins.
RESULTS
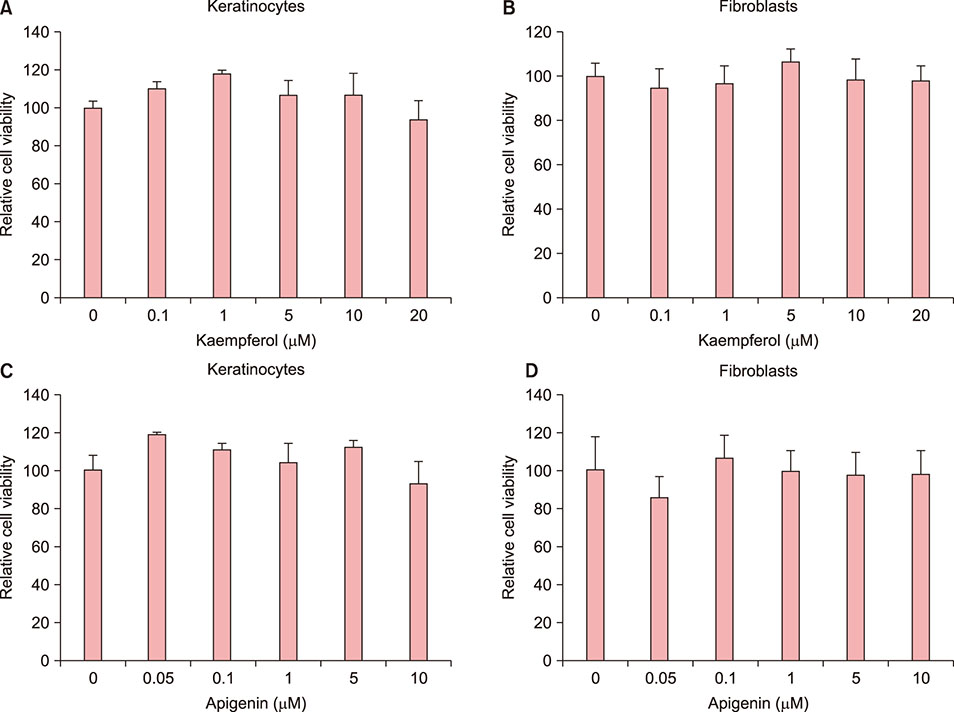
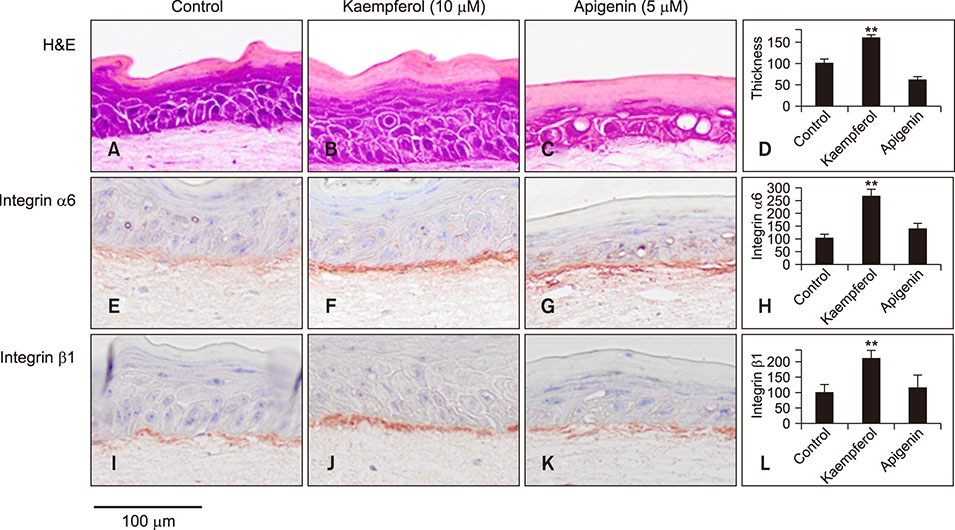
Kaempferol increased the thickness of the epidermis when added to prepare SEs. In addition, the basal cells of kaempferol- treated SEs appeared more columnar. In the immunohistological study, the expression of integrins α6 and β1 and the numbers of p63- and PCNA-positive cells were markedly higher in the kaempferol-treated model. However, apigenin showed no effects on the formation of three-dimensional skin models. RT-PCR analysis also confirmed that kaempferol increased the expression of integrin α6 and integrin β1.
CONCLUSION
Our findings indicated that kaempferol can increase the proliferative potential of basal epidermal cells by modulating the basement membrane. In other words, kaempferol can affect the fate of interfollicular epidermal stem cells by increasing the expression of both integrins α6 and β1. These effects, in particular, might be ascribed to the 3-OH group of kaempferol.
Keyword
MeSH Terms
Figure
Reference
-
1. Mustafa RA, Abdul Hamid A, Mohamed S, Bakar FA. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J Food Sci. 2010; 75:C28–C35.
Article2. Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011; 11:298–344.
Article3. Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013; 138:2099–2107.
Article4. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000; 52:673–751.5. Luo C, Yang H, Tang C, Yao G, Kong L, He H, et al. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int Immunopharmacol. 2015; 28:744–750.
Article6. Kim M, Lim SJ, Kang SW, Um BH, Nho CW. Aceriphyllum rossii extract and its active compounds, quercetin and kaempferol inhibit IgE-mediated mast cell activation and passive cutaneous anaphylaxis. J Agric Food Chem. 2014; 62:3750–3758.
Article7. El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002; 11:398–405.
Article8. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997; 337:1419–1428.
Article9. Amano S. Possible involvement of basement membrane damage in skin photoaging. J Investig Dermatol Symp Proc. 2009; 14:2–7.
Article10. Kaur P, Li A. Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Invest Dermatol. 2000; 114:413–420.
Article11. Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011; 3:pii: a005124.
Article12. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996; 20:933–956.
Article13. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975; 6:331–343.
Article14. Choi HR, Park SH, Choi JW, Kim DS, Park KC. A simple assay method for melanosome transfer. Ann Dermatol. 2012; 24:90–93.
Article15. Kim DS, Cho HJ, Choi HR, Kwon SB, Park KC. Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell Mol Life Sci. 2004; 61:2774–2781.
Article16. Janes SM, Lowell S, Hutter C. Epidermal stem cells. J Pathol. 2002; 197:479–491.
Article17. Li ER, Owens DM, Djian P, Watt FM. Expression of involucrin in normal, hyperproliferative and neoplastic mouse keratinocytes. Exp Dermatol. 2000; 9:431–438.
Article18. Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001; 49:3106–3112.
Article19. Choi HR, Byun SY, Kwon SH, Park KC. Niche interactions in epidermal stem cells. World J Stem Cells. 2015; 7:495–501.
Article20. Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987; 326:515–517.
Article21. Choi JW, Nam KM, Choi HR, Lee DH, Huh CH, Park KC. Decreased Galectin-3 and -7 expressions in old-aged skin and their differential expression in skin equivalents. Ann Dermatol. 2018; 30:375–378.
Article22. Chae JB, Yang SH, Byun SY, Choi HR, Shin JW, Park KC. The effects of hydroporation on melasma with anti-aging cocktail. J Cosmet Dermatol. 2017; 16:e15–e20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum: The Effects of the 3-OH Group of Kaempferol on Interfollicular Epidermal Stem Cell Fate
- Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells
- Regulation of Neural Stem Cell Fate by Natural Products
- Anti-cancer Effect and Underlying Mechanism(s) of Kaempferol, a Phytoestrogen, on the Regulation of Apoptosis in Diverse Cancer Cell Models
- Chemicals as the Sole Transformers of Cell Fate