Allergy Asthma Immunol Res.
2018 Jan;10(1):25-33. 10.4168/aair.2018.10.1.25.
Alteration in Claudin-4 Contributes to Airway Inflammation and Responsiveness in Asthma
- Affiliations
-
- 1Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. jas877@schmc.ac.kr
- 2Department of Environmental and Occupational Health, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
- KMID: 2428847
- DOI: http://doi.org/10.4168/aair.2018.10.1.25
Abstract
- PURPOSE
Claudin-4 has been reported to function as a paracellular sodium barrier and is one of the 3 major claudins expressed in lung alveolar epithelial cells. However, the possible role of claudin-4 in bronchial asthma has not yet been fully studied. In this study, we aimed to elucidate the role of claudin-4 in the pathogenesis of bronchial asthma.
METHODS
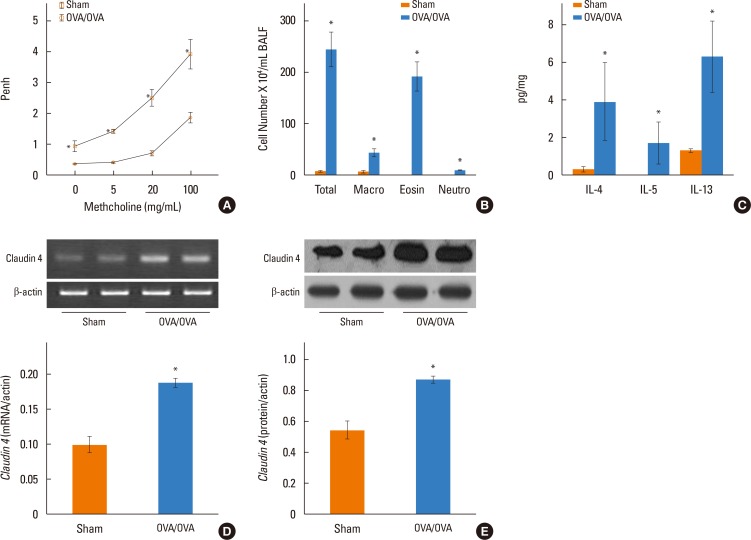
We determined claudin-4 levels in blood from asthmatic patients. Moreover, using mice sensitized and challenged with OVA, as well as sensitized and challenged with saline, we investigated whether claudin-4 is involved in the pathogenesis of bronchial asthma. Der p1 induced the inflammatory cytokines in NHBE cells.
RESULTS
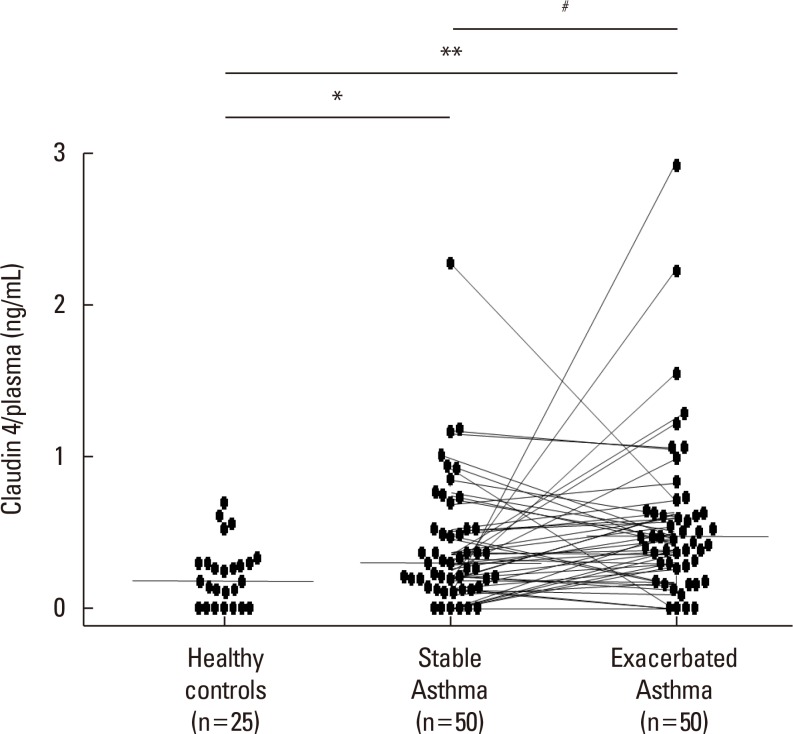
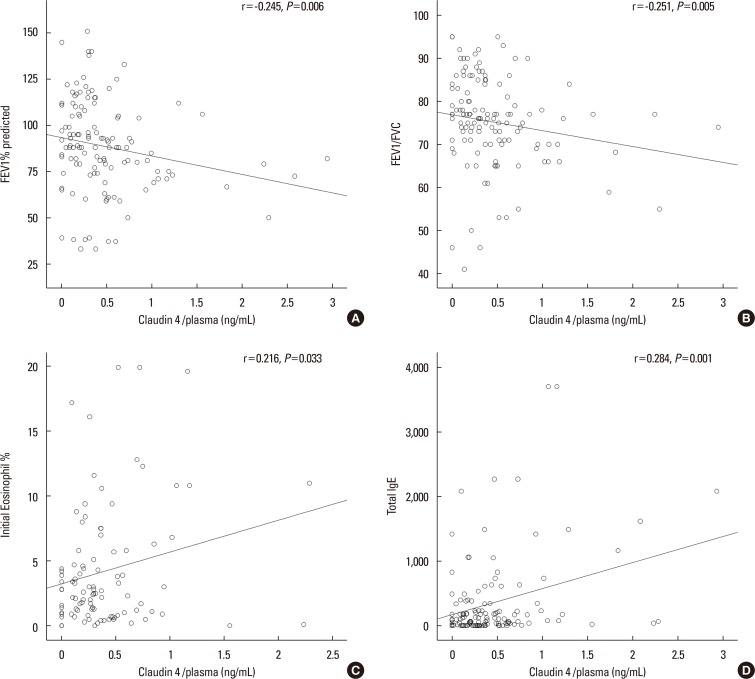
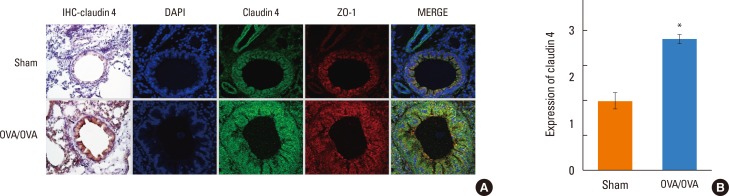
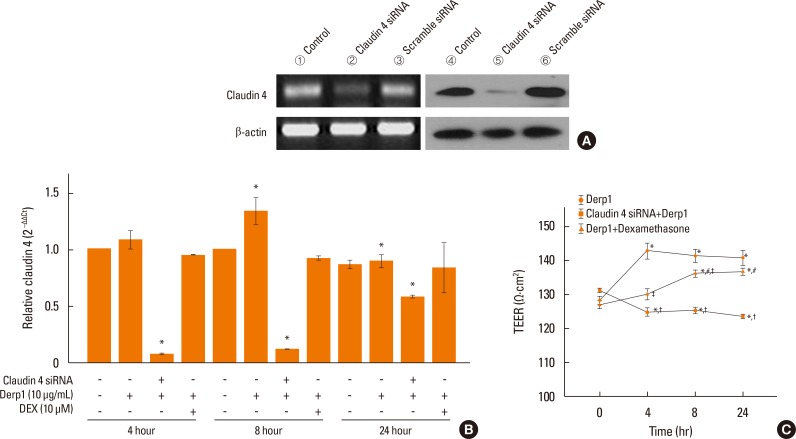
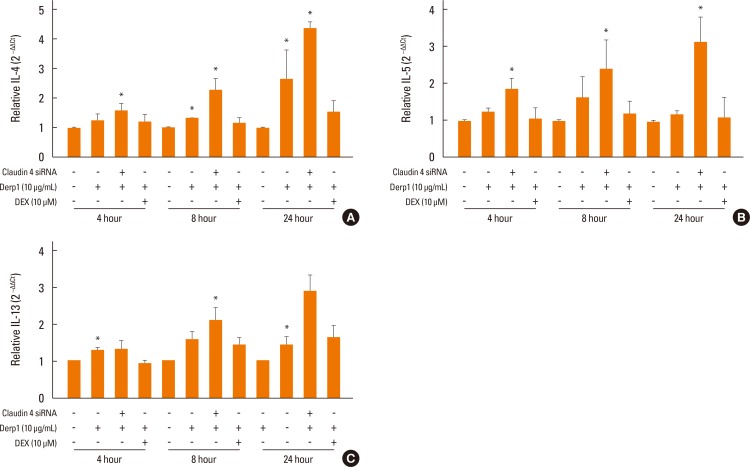
We found that claudin-4 in blood from asthmatic patients was increased compared with that from healthy control subjects. Plasma claudin-4 levels were significantly higher in exacerbated patients than in control patients with bronchial asthma. The plasma claudin-4 level was correlated with eosinophils, total IgE, FEV1% pred, and FEV1/FVC. Moreover, lung tissues from the OVA-OVA mice showed significant increases in transcripts and proteins of claudin-4 as well as in TJ breaks and the densities of claudin-4 staining. When claudin-4 was knocked down by transfecting its siRNA, inflammatory cytokine expressions, which were induced by Der p1 treatment, were significantly increased.
CONCLUSIONS
These findings thus raise the possibility that regulation of lung epithelial barrier proteins may constitute a therapeutic approach for asthma.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Impact of the Endothelial Tight Junction Protein Claudin-5 on Clinical Profiles of Patients With COPD
Byeong-Gon Kim, Pureun-Haneul Lee, Sun-Hye Lee, Ae-Rin Baek, Jong-Sook Park, Junehyuk Lee, Sung-Woo Park, Do-Jin Kim, Choon-Sik Park, An-Soo Jang
Allergy Asthma Immunol Res. 2018;10(5):533-542. doi: 10.4168/aair.2018.10.5.533.
Reference
-
1. Harkness LM, Ashton AW, Burgess JK. Asthma is not only an airway disease, but also a vascular disease. Pharmacol Ther. 2015; 148:17–33. PMID: 25460035.
Article2. Global Initiative for Asthma. Global strategy for asthma management and prevention 2014 [Internet]. [place unknown]: Global Initiative for Asthma;2014. cited 2016 Jun 3. Available from: http://www.ginasthma.org/.3. Park HW, Tantisira KG. Genetic signatures of asthma exacerbation. Allergy Asthma Immunol Res. 2017; 9:191–199. PMID: 28293925.
Article4. Bossé Y, Paré PD, Seow CY. Airway wall remodeling in asthma: from the epithelial layer to the adventitia. Curr Allergy Asthma Rep. 2008; 8:357–366. PMID: 18606090.
Article5. Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003; 167:1360–1368. PMID: 12531777.
Article6. Ebina M, Yaegashi H, Chiba R, Takahashi T, Motomiya M, Tanemura M. Hyperreactive site in the airway tree of asthmatic patients revealed by thickening of bronchial muscles. A morphometric study. Am Rev Respir Dis. 1990; 141:1327–1332. PMID: 2187387.
Article7. Wilson J. The bronchial microcirculation in asthma. Clin Exp Allergy. 2000; 30(Suppl 1):51–53. PMID: 10849476.
Article8. Davies DE. Epithelial barrier function and immunity in asthma. Ann Am Thorac Soc. 2014; 11(Suppl 5):S244–S251. PMID: 25525727.
Article9. Park CS, Kim YY, Kang SY. Collection between RAST and skin test for inhalant offending allergens. Allergy. 1983; 3:1–9.10. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006; 355:763–778. PMID: 16926275.
Article11. Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012; 18:705–715. PMID: 22561834.
Article12. Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004; 4:978–988. PMID: 15573132.
Article13. Eiwegger T, Akdis CA. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur J Immunol. 2011; 41:1535–1538. PMID: 21618506.
Article14. Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009; 15:410–416. PMID: 19330007.
Article15. Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014; 134:509–520. PMID: 25085341.
Article16. Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007; 120:1279–1284. PMID: 17949801.17. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001; 2:285–293. PMID: 11283726.
Article18. Chen X, Gumbiner BM. Crosstalk between different adhesion molecules. Curr Opin Cell Biol. 2006; 18:572–578. PMID: 16859906.
Article19. Reijula JP, Reijula KE. The impact of Finnish tobacco legislation on restaurant workers’ exposure to tobacco smoke at work. Scand J Public Health. 2010; 38:724–730. PMID: 20688793.
Article21. Kage H, Flodby P, Gao D, Kim YH, Marconett CN, DeMaio L, et al. Claudin 4 knockout mice: normal physiological phenotype with increased susceptibility to lung injury. Am J Physiol Lung Cell Mol Physiol. 2014; 307:L524–L536. PMID: 25106430.
Article22. Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol. 2013; 75:551–567. PMID: 23072447.
Article23. Jin W, Rong L, Liu Y, Song Y, Li Y, Pan J. Increased claudin-3, -4 and -18 levels in bronchoalveolar lavage fluid reflect severity of acute lung injury. Respirology. 2013; 18:643–651. PMID: 23253121.
Article24. Lappi-Blanco E, Lehtonen ST, Sormunen R, Merikallio HM, Soini Y, Kaarteenaho RL. Divergence of tight and adherens junction factors in alveolar epithelium in pulmonary fibrosis. Hum Pathol. 2013; 44:895–907. PMID: 23253490.
Article25. Moon KY, Lee PH, Kim BG, Park CS, Leikauf GD, Jang AS. Claudin 5 in a murine model of allergic asthma: its implication and response to steroid treatment. J Allergy Clin Immunol. 2015; 136:1694–1696.e5. PMID: 26409663.
Article26. Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015; 46:622–639. PMID: 26206872.
Article27. Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015; 43:29–40. PMID: 26200011.
Article28. Schlingmann B, Molina SA, Koval M. Claudins: gatekeepers of lung epithelial function. Semin Cell Dev Biol. 2015; 42:47–57. PMID: 25951797.
Article29. Chae MC, Park CK, Keum DY, Hwang I, Kwon KY, Jang BC. Prognostic significance of claudin 4 in completely resected adenocarcinoma of the lung. Korean J Thorac Cardiovasc Surg. 2014; 47:262–268. PMID: 25207224.
Article30. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999; 104:123–133. PMID: 10393706.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- N-acetylcysteine decreases airway inflammation and responsiveness in asthma by modulating claudin 18 expression
- Serum eosinophil cationic protein(ECP) in children with atopic asthma
- Evolution of Asthma Concept and Effect of Current Asthma Management Guidelines
- Inhaled Steroids in Asthma Therapy: An Update
- Airway remodelling in asthma: role for mechanical forces