Korean J Ophthalmol.
2018 Dec;32(6):470-477. 10.3341/kjo.2018.0015.
Relationship between Progressive Changes in Lamina Cribrosa Depth and Deterioration of Visual Field Loss in Glaucomatous Eyes
- Affiliations
-
- 1Department of Ophthalmology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sungeye@gmail.com
- 2Department of Ophthalmology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- KMID: 2427961
- DOI: http://doi.org/10.3341/kjo.2018.0015
Abstract
- PURPOSE
To investigate the relationship between the progression of visual field (VF) loss and changes in lamina cribrosa depth (LCD) as determined by spectral-domain optical coherence tomography (SD-OCT) enhanced depth imaging in patients with primary open angle glaucoma (POAG).
METHODS
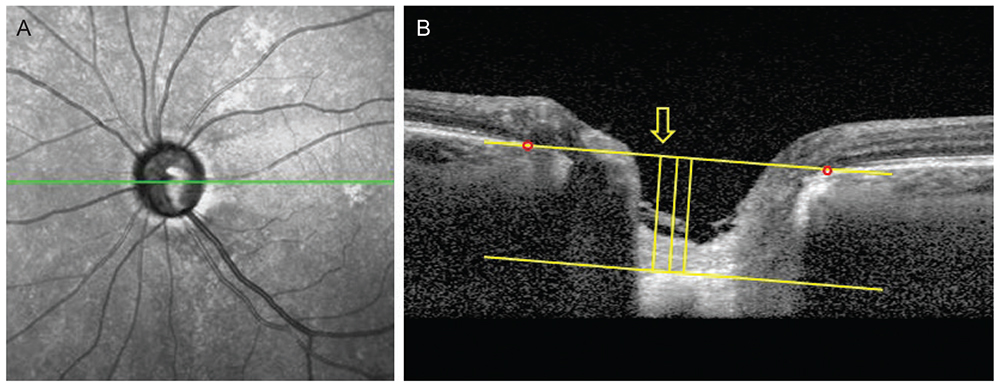
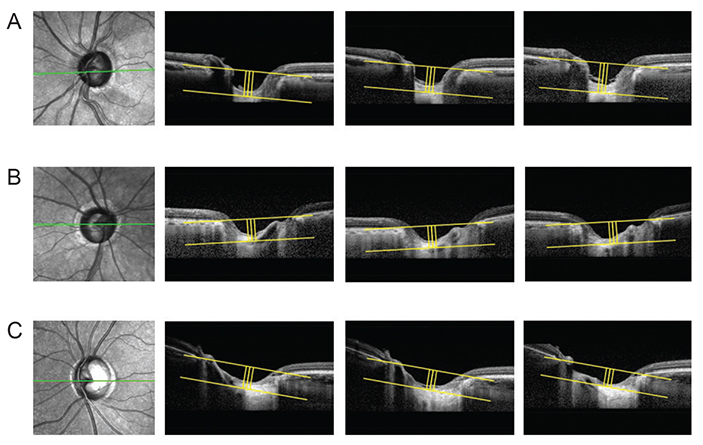
Data from 60 POAG patients (mean follow-up, 3.5 ± 0.7 years) were included in this retrospective study. The LCD was measured in the optic disc image using SD-OCT enhanced depth imaging scanning at each visit. Change in the LCD was considered to either "˜increase' or "˜decrease' when the differences between baseline and the latest two consecutive follow-up visits were greater than the corresponding reproducibility coefficient value (23.08 µm, as determined in a preliminary reproducibility study). All participants were divided into three groups: increased LCD (ILCD), decreased LCD (DLCD), and no LCD change (NLCD). The Early Manifest Glaucoma Trial criteria were used to define VF deterioration. Kaplan-Meier survival analysis and Cox's proportional hazard models were performed to explore the relationship between VF progression and LCD change.
RESULTS
Of the 60 eyes examined, 35.0% (21 eyes), 28.3% (17 eyes), and 36.7% (22 eyes) were classified as the ILCD, DLCD, and NLCD groups, respectively. Kaplan-Meier survival analysis showed a greater cumulative probability of VF progression in the ILCD group than in the NLCD (p < 0.001) or DLCD groups (p = 0.018). Increased LCD was identified as the only risk factor for VF progression in the Cox proportional hazard models (hazard ratio, 1.008; 95% confidence interval, 1.000 to 1.015; p = 0.047).
CONCLUSIONS
Increased LCD was associated with a greater possibility of VF progression. The quantitative measurement of LCD changes, determined by SD-OCT, is a potential biomarker for the prediction of VF deterioration in patients with POAG.
MeSH Terms
Figure
Reference
-
1. Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981; 99:635–649.2. Quigley HA, Anderson DR. Distribution of axonal transport blockade by acute intraocular pressure elevation in the primate optic nerve head. Invest Ophthalmol Vis Sci. 1977; 16:640–644.3. Radius RL, Anderson DR. Rapid axonal transport in primate optic nerve. Distribution of pressure-induced interruption. Arch Ophthalmol. 1981; 99:650–654.4. Kim TW, Kagemann L, Girard MJ, et al. Imaging of the lamina cribrosa in glaucoma: perspectives of pathogenesis and clinical applications. Curr Eye Res. 2013; 38:903–909.
Article5. Nadler Z, Wang B, Wollstein G, et al. Repeatability of in vivo 3D lamina cribrosa microarchitecture using adaptive optics spectral domain optical coherence tomography. Biomed Opt Express. 2014; 5:1114–1123.
Article6. Vilupuru AS, Rangaswamy NV, Frishman LJ, et al. Adaptive optics scanning laser ophthalmoscopy for in vivo imaging of lamina cribrosa. J Opt Soc Am A Opt Image Sci Vis. 2007; 24:1417–1425.
Article7. Chung HS, Sung KR, Lee KS, et al. Relationship between the lamina cribrosa, outer retina, and choroidal thickness as assessed using spectral domain optical coherence tomography. Korean J Ophthalmol. 2014; 28:234–240.
Article8. Burgoyne CF, Downs JC. Premise and prediction-how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head. J Glaucoma. 2008; 17:318–328.
Article9. Yang H, Downs JC, Bellezza A, et al. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: prelaminar neural tissues and cupping. Invest Ophthalmol Vis Sci. 2007; 48:5068–5084.
Article10. Furlanetto RL, Park SC, Damle UJ, et al. Posterior displacement of the lamina cribrosa in glaucoma: in vivo interindividual and intereye comparisons. Invest Ophthalmol Vis Sci. 2013; 54:4836–4842.
Article11. Xu G, Weinreb RN, Leung CK. Optic nerve head deformation in glaucoma: the temporal relationship between optic nerve head surface depression and retinal nerve fiber layer thinning. Ophthalmology. 2014; 121:2362–2370.12. Lee EJ, Kim TW, Kim M, Kim H. Influence of lamina cribrosa thickness and depth on the rate of progressive retinal nerve fiber layer thinning. Ophthalmology. 2015; 122:721–729.
Article13. Omodaka K, Takahashi S, Matsumoto A, et al. Clinical factors associated with lamina cribrosa thickness in patients with glaucoma, as measured with swept source optical coherence tomography. PLoS One. 2016; 11:e0153707.
Article14. Chung HS, Sung KR, Lee JY, Na JH. Lamina cribrosa-related parameters assessed by optical coherence tomography for prediction of future glaucoma progression. Curr Eye Res. 2016; 41:806–813.
Article15. Lee EJ, Kim TW, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012; 119:1359–1366.
Article16. Kim S, Sung KR, Lee JR, Lee KS. Evaluation of lamina cribrosa in pseudoexfoliation syndrome using spectral-domain optical coherence tomography enhanced depth imaging. Ophthalmology. 2013; 120:1798–1803.
Article17. Vaz S, Falkmer T, Passmore AE, et al. The case for using the repeatability coefficient when calculating test-retest reliability. PLoS One. 2013; 8:e73990.
Article18. Heijl A, Leske MC, Bengtsson B, et al. Measuring visual field progression in the Early Manifest Glaucoma Trial. Acta Ophthalmol Scand. 2003; 81:286–293.
Article19. Lee EJ, Kim TW, Weinreb RN, et al. Three-dimensional evaluation of the lamina cribrosa using spectral-domain optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2012; 53:198–204.
Article20. Bellezza AJ, Rintalan CJ, Thompson HW, et al. Anterior scleral canal geometry in pressurised (IOP 10) and non-pressurised (IOP 0) normal monkey eyes. Br J Ophthalmol. 2003; 87:1284–1290.
Article21. Vianna JR, Lanoe VR, Quach J, et al. Serial changes in lamina cribrosa depth and neuroretinal parameters in glaucoma: impact of choroidal thickness. Ophthalmology. 2017; 124:1392–1402.22. Faridi OS, Park SC, Kabadi R, et al. Effect of focal lamina cribrosa defect on glaucomatous visual field progression. Ophthalmology. 2014; 121:1524–1530.
Article23. Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977; 16:426–441.24. Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981; 99:137–143.
Article25. Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Predicted extension, compression and shearing of optic nerve head tissues. Exp Eye Res. 2007; 85:312–322.
Article26. Reis AS, O'Leary N, Stanfield MJ, et al. Laminar displacement and prelaminar tissue thickness change after glaucoma surgery imaged with optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:5819–5826.
Article27. Lee EJ, Kim TW, Weinreb RN, Kim H. Reversal of lamina cribrosa displacement after intraocular pressure reduction in open-angle glaucoma. Ophthalmology. 2013; 120:553–559.
Article28. Sigal IA, Yang H, Roberts MD, et al. IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models. Invest Ophthalmol Vis Sci. 2011; 52:1896–1907.
Article29. Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011; 52:5121–5129.
Article30. Rho CR, Park HY, Lee NY, Park CK. Clock-hour laminar displacement and age in primary open-angle glaucoma and normal tension glaucoma. Clin Exp Ophthalmol. 2012; 40:e183–e189.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lamina Cribrosa Thickness in the Fellow Eyes of Patients with Unilateral Retinal Vein Occlusion
- Inter-eye Comparison of the Lamina Cribrosa Depth in Patients with Bilateral Normal-tension Glaucoma with Asymmetrical Damage
- Early Postoperative Visual Acuity and Visual Field Change Following Filtration Surgery in Advanced Glaucomatous Damaged Eyes
- Measurement of Deep Optic Nerve Complex Structures with Two Spectral Domain Optical Coherence Tomography Instruments
- Sudden Wipeout Phenomenon Following Fitering Surgery in Patients with Advanced Glaucoma