Chonnam Med J.
2018 Sep;54(3):135-142. 10.4068/cmj.2018.54.3.135.
Melanoma Cell Death Mechanisms
- Affiliations
-
- 1Department of Dermatology, University of Colorado Denver School of Medicine, Aurora, CO, USA. peter.song@ucdenver.edu
- 2Department of Internal Medicine, University of Colorado Denver School of Medicine, Aurora, CO, USA.
- 3Marian University College of Osteopathic Medicine, Indianapolis, IN, USA.
- KMID: 2420880
- DOI: http://doi.org/10.4068/cmj.2018.54.3.135
Abstract
- Over recent years, several new molecular and immunogenic therapeutic approaches to melanoma treatment have been approved and implemented in clinical practice. Mechanisms of resistance to these new therapies have become a major problem. Mutation-specific pharmacotherapy can result in simultaneous emergence of resistant clones at many separate body sites despite an initially positive therapeutic response. Additionally, treatments aimed at inducing apoptosis are subject to resistance due to escape through other known mechanisms of regulated cell death (RCD). In this review, we discuss the complexity in pharmacological manipulation of melanoma with c-Kit, BRAF, MEK, and/or mTOR mutant cell lines. This study also addresses melanoma evasion of cell death through modalities of RCD such as apoptosis, autophagy, and necroptosis. This study also examines new combination therapies which have been approved to target both cell cycle dysregulation and cell death pathways. Lastly, we recognize the importance of immunomodulation though manipulation of the body's natural killing mechanisms with CTLA4, PD1, and CSF1 inhibition. As we begin to recognize tumor cell activation of alternate pathways, evasion of programmed cell death, and manipulation of the tumor microenvironment, it is increasingly important to grasp the complexity of personalized therapy in melanoma treatment.
MeSH Terms
Figure
Reference
-
1. Mattia G, Puglisi R, Ascione B, Malorni W, Carè A, Matarrese P. Cell death-based treatments of melanoma:conventional treatments and new therapeutic strategies. Cell Death Dis. 2018; 9:112.
Article2. Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016; 136:1161–1171.
Article3. Carvajal RD. Targeting KIT for treatment of advanced melanoma. Melanoma Lett Winter. 2011; 29.4. Amann VC, Ramelyte E, Thurneysen S, Pitocco R, Bentele-Jaberg N, Goldinger SM, et al. Developments in targeted therapy in melanoma. Eur J Surg Oncol. 2017; 43:581–593.
Article5. Smalley KS, Sondak VK. Melanoma--an unlikely poster child for personalized cancer therapy. N Engl J Med. 2010; 363:876–878.
Article6. Cheng L, Lopez-Beltran A, Massari F, MacLennan GT, Montironi R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Mod Pathol. 2018; 31:24–38.
Article7. Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017; 545:175–180.
Article8. Moon KR, Choi YD, Kim JM, Jin S, Shin MH, Shim HJ, et al. Genetic alterations in primary acral melanoma and acral melanocytic nevus in Korea: common mutated genes show distinct cytomorphological features. J Invest Dermatol. 2018; 138:933–945.
Article9. Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013; 31:3182–3190.
Article10. Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011; 29:2904–2909.
Article11. Gounder MM, Maki RG. Molecular basis for primary and secondary tyrosine kinase inhibitor resistance in gastrointestinal stromal tumor. Cancer Chemother Pharmacol. 2011; 67:Suppl 1. S25–S43.
Article12. Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010; 70:5213–5219.
Article13. Manzano JL, Layos L, Bugés C, de Los Llanos Gil M, Vila L, Martínez-Balibrea E, et al. Resistant mechanisms to BRAF inhibitors in melanoma. Ann Transl Med. 2016; 4:237.
Article14. Ryu S, Youn C, Moon AR, Howland A, Armstrong CA, Song PI. Therapeutic inhibitors against mutated BRAF and MEK for the treatment of metastatic melanoma. Chonnam Med J. 2017; 53:173–177.
Article15. Welsh SJ, Rizos H, Scolyer RA, Long GV. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: where to next? Eur J Cancer. 2016; 62:76–85.
Article16. Grimaldi AM, Simeone E, Festino L, Vanella V, Strudel M, Ascierto PA. MEK inhibitors in the treatment of metastatic melanoma and solid tumors. Am J Clin Dermatol. 2017; 18:745–754.
Article17. Lugowska I, Koseła-Paterczyk H, Kozak K, Rutkowski P. Trametinib: a MEK inhibitor for management of metastatic melanoma. Onco Targets Ther. 2015; 8:2251–2259.18. Cobimetinib for metastatic melanoma. Aust Prescr. 2017; 40:30–31.19. Kim DW, Patel SP. Profile of selumetinib and its potential in the treatment of melanoma. Onco Targets Ther. 2014; 7:1631–1639.20. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015; 386:444–451.
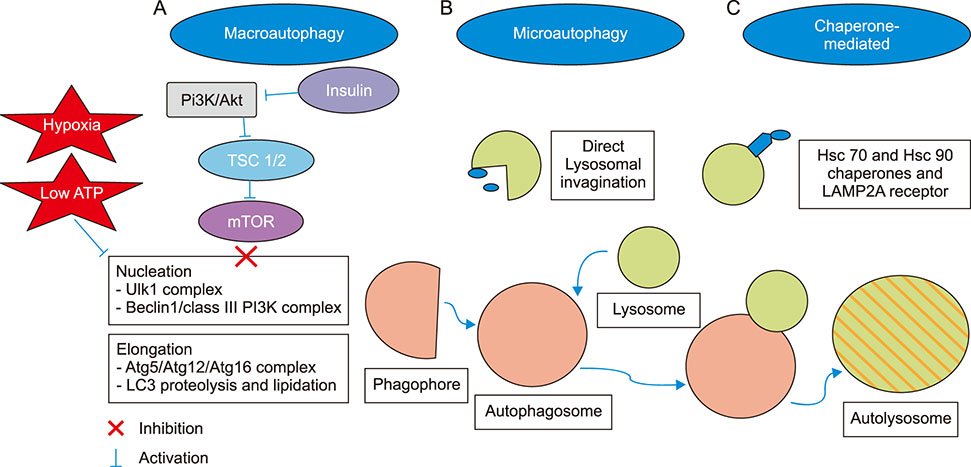
Article21. Karbowniczek M, Spittle CS, Morrison T, Wu H, Henske EP. MTOR is activated in the majority of malignant melanomas. J Invest Dermatol. 2008; 128:980–987.
Article22. Kong Y, Si L, Li Y, Wu X, Xu X, Dai J, et al. Analysis of mTOR gene aberrations in melanoma patients and evaluation of their sensitivity to PI3K-AKT-mTOR pathway inhibitors. Clin Cancer Res. 2016; 22:1018–1027.
Article23. Vera Aguilera J, Rao RD, Allred JB, Suman VJ, Windschitl HE, Kaur JS, et al. Phase II study of Everolimus in metastatic malignant melanoma (NCCTG-N0377, alliance). Oncologist. 2018; 23:887–e94.
Article24. Hirayama Y, Gi M, Yamano S, Tachibana H, Okuno T, Tamada S, et al. Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci. 2016; 107:1736–1744.
Article25. Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011; 104:643–652.
Article26. Xie X, White EP, Mehnert JM. Coordinate autophagy and mTOR pathway inhibition enhances cell death in melanoma. PLoS One. 2013; 8:e55096.
Article27. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on Cell death 2018. Cell Death Differ. 2018; 25:486–541.28. Adewale FO, Basiru AO, Ayorinde OO, Israel OI, Oluwafemi OA. Regulation of apoptotic and necroptotic cell death in skin cancer. J Cancer Biol Res. 2017; 5:1108–1117.29. Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011; 30:87.
Article30. Avery-Kiejda KA, Bowden NA, Croft AJ, Scurr LL, Kairupan CF, Ashton KA, et al. P53 in human melanoma fails to regulate target genes associated with apoptosis and the cell cycle and may contribute to proliferation. BMC Cancer. 2011; 11:203.
Article31. Vucic D, Stennicke HR, Pisabarro MT, Salvesen GS, Dixit VM. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr Biol. 2000; 10:1359–1366.
Article32. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010; 221:3–12.
Article33. Duffy A, Le J, Sausville E, Emadi A. Autophagy modulation: a target for cancer treatment development. Cancer Chemother Pharmacol. 2015; 75:439–447.
Article34. Cheng Y, Ren X, Hait WN, Yang JM. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev. 2013; 65:1162–1197.
Article35. Egger ME, Huang JS, Yin W, McMasters KM, McNally LR. Inhibition of autophagy with chloroquine is effective in melanoma. J Surg Res. 2013; 184:274–281.
Article36. Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013; 38:209–223.
Article37. Geserick P, Wang J, Schilling R, Horn S, Harris PA, Bertin J, et al. Absence of RIPK3 predicts necroptosis resistance in malignant melanoma. Cell Death Dis. 2015; 6:e1884.
Article38. Caldarola G, Carbone A, Arena V, Pennacchia I, De Waure C, Vianale G, et al. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL): a possible pathogenic role in chronic plaque psoriasis. G Ital Dermatol Venereol. 2016; 151:17–24.39. Karlsson AK, Saleh SN. Checkpoint inhibitors for malignant melanoma: a systematic review and meta-analysis. Clin Cosmet Investig Dermatol. 2017; 10:325–339.
Article40. Neubert NJ, Schmittnaegel M, Bordry N, Nassiri S, Wald N, Martignier C, et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Transl Med. 2018; 10:eaan3311.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell Death and Bacterial Infection
- Growth and Characterization of the Uveal Melanoma in Vitro
- Alterations of the Apoptosis Genes and Their Products in Non-small Cell Lung Cancer Tissues
- Activation of transforming growth factor-β and epithelialmesenchymal transition enhance regulatory T cells-mediated metastasis
- A Case of Malignant Melanoma of Soft Parts with Unusual Histopathologic Findings