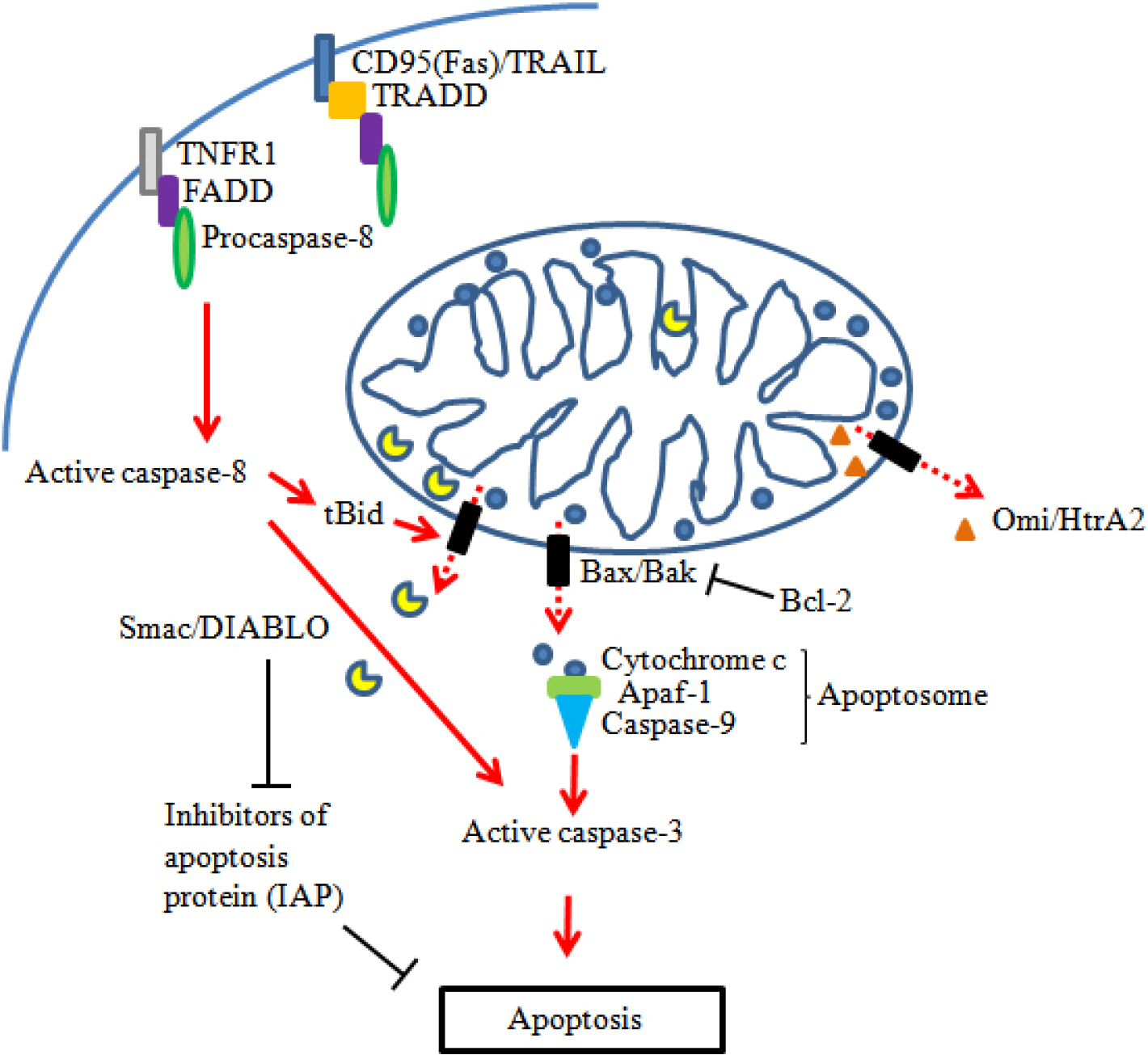
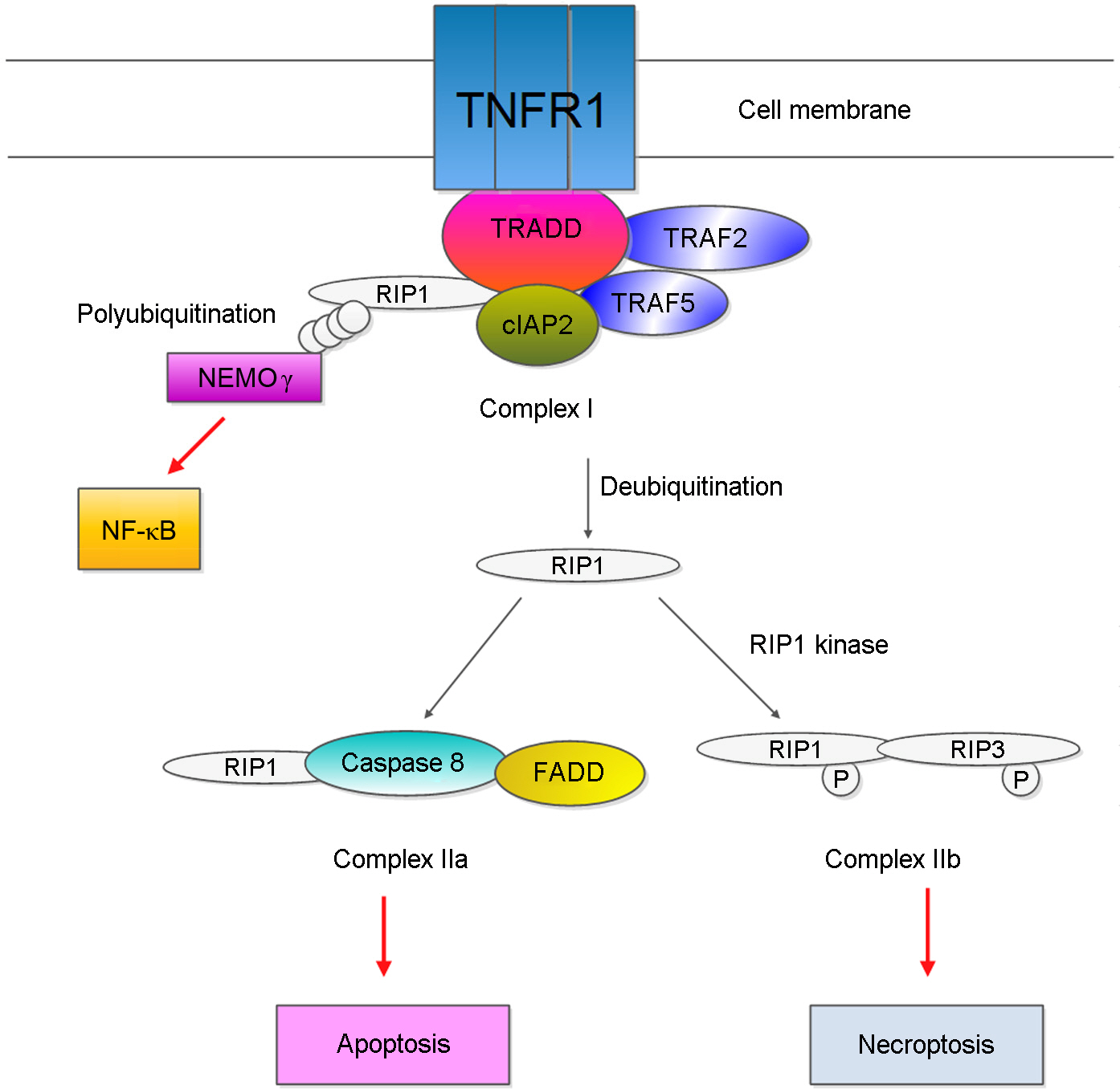
J Bacteriol Virol.
2013 Jun;43(2):85-91. 10.4167/jbv.2013.43.2.85.
Cell Death and Bacterial Infection
- Affiliations
-
- 1Department of Microbiology and Research Institute for Medical Sciences, College of Medicine, Chungnam National University, Daejeon, Korea. songch@cnu.ac.kr
- KMID: 2168677
- DOI: http://doi.org/10.4167/jbv.2013.43.2.85
Abstract
- Cells can die through various biochemical pathways related to complex pathophysiological process. Different types of cell death are closely associated with microbial infection. Several regulatory mechanisms of cell death during bacterial infection play important roles to control the pathogens. Bacteria usually manipulate host defense mechanisms to survive and eventually replicate. Host cell death is one of the intrinsic immune defense mechanisms even if infected cells were sacrificed and it induced detrimental effects on host. Understanding the role of cell death during bacterial infection is important to provide insight into the pathogenesis of unknown infectious diseases. In this review, the different forms of cell death are discussed.
Keyword
Figure
Cited by 1 articles
-
Mycobacterium bovis Bacillus Calmette-Guerin (BCG) and BCG-based Vaccines Against Tuberculosis
Seung Bin Cha, Sung Jae Shin
J Bacteriol Virol. 2014;44(3):236-243. doi: 10.4167/jbv.2014.44.3.236.
Reference
-
1). Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972; 26:239–57.
Article2). Zychlinsky A, Sansonetti P. Perspectives series: host/pathogen interactions. Apoptosis in bacterial pathogenesis. J Clin Invest. 1997; 100:493–5.
Article3). Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005; 73:1907–16.
Article4). Gao L, Abu Kwaik Y. Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect. 2000; 2:1705–19.5). Weinrauch Y, Zychlinsky A. The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol. 1999; 53:155–87.
Article6). Hay S, Kannourakis G. A time to kill: viral manipulation of the cell death program. J Gen Virol. 2002; 83:1547–64.
Article7). Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010; 189:1059–70.
Article8). Torchinsky MB, Garaude J, Blander JM. Infection and apoptosis as a combined inflammatory trigger. Curr Opin Immunol. 2010; 22:55–62.
Article9). Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009; 9:353–63.
Article10). Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994; 263:678–81.11). Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med. 1994; 180:1499–509.
Article12). Behar SM, Martin CJ, Nunes-Alves C, Divangahi M, Remold HG. Lipids, apoptosis, and cross-presentation: links in the chain of host defense against Mycobacterium tuberculosis. Microbes Infect. 2011; 13:749–56.13). Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol. 2010; 11:751–8.14). Song CH. Endoplasmic Reticulum Stress Responses and Apoptosis. J Bacteriol Virol. 2012; 42:196–202.
Article15). Lee EJ, Cho JA, Seong SY. Cell Death and Immunity. J Bacteriol Virol. 2011; 41:309–11.
Article16). Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516.
Article17). Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002; 2:277–88.
Article18). Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000; 403:98–103.19). Kang SJ, Wang S, Kuida K, Yuan J. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ. 2002; 9:1115–25.
Article20). Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997; 272:32401–10.
Article21). Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001; 20:2122–33.
Article22). Peter ME, Krammer PH. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998; 10:545–51.
Article23). Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995; 81:495–504.24). Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002; 296:1635–6.
Article25). Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006; 13:1423–33.
Article26). Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000; 102:33–42.
Article27). Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006; 20:1–15.
Article28). Hetz CA, Torres V, Quest AF. Beyond apoptosis: nonapoptotic cell death in physiology and disease. Biochem Cell Biol. 2005; 83:579–88.
Article29). Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000; 1:489–95.
Article30). Fiers W, Beyaert R, Boone E, Cornelis S, Declercq W, Decoster E, et al. TNF-induced intracellular signaling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J Inflamm. 1995; 47:67–75.31). Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008; 135:1311–23.
Article32). Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012; 82:249–58.
Article33). Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008; 105:11778–83.34). Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009; 138:229–32.
Article35). Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010; 22:263–8.
Article36). Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012; 13:954–62.37). Roca FJ, Ramakrishnan L. TNF Dually Mediates Resistance and Susceptibility to Mycobacteria via Mitochondrial Reactive Oxygen Species. Cell. 2013; 153:521–34.
Article38). Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009; 7:99–109.
Article39). Frantz S, Ducharme A, Sawyer D, Rohde LE, Kobzik L, Fukazawa R, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003; 35:685–94.
Article40). Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006; 8:1812–25.
Article41). Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008; 3:224–32.42). Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozören N, Brady G, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006; 281:35217–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Septic Knee Arthritis Caused by Group B Salmonella Species in a Patient with Systemic Lupus Erythematosus
- Analysis on the Causes of Death After Neutropenic Fever Episodes in Pediatric Cancer Patients
- Cell Death and Immunity
- Gas Forming Bacterial Infection after Total Knee Arthroplasty
- Cellular Bronchoalveolar Lavage Profile Following Induced Bacterial Infection and Rejection of Lung Allografts