Yonsei Med J.
2017 May;58(3):570-580. 10.3349/ymj.2017.58.3.570.
Optimal Dose and Timing of Umbilical Stem Cells Treatment in Pulmonary Arterial Hypertensive Rats
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea. ymhong@ewha.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Biomedical Research Institute, MEDIPOST, Co., Seoul, Korea.
- KMID: 2419115
- DOI: http://doi.org/10.3349/ymj.2017.58.3.570
Abstract
- PURPOSE
Pulmonary arterial hypertension (PAH) is a fatal disease which is characterized by an increase in pulmonary arterial pressure leading to increases in right ventricular afterload. Human umbilical cord blood derived-mesenchymal stem cells (hUCB-MSCs) administered via the jugular vein have been previously shown to improve PAH by reversal treatment. However, the effect of low dosage and transfusion timing of hUCB-MSCs on PAH has not yet been clearly established. Obviously, low dosage treatment can lead to a reduction in costs. This is the first study on early transfusion effect.
MATERIALS AND METHODS
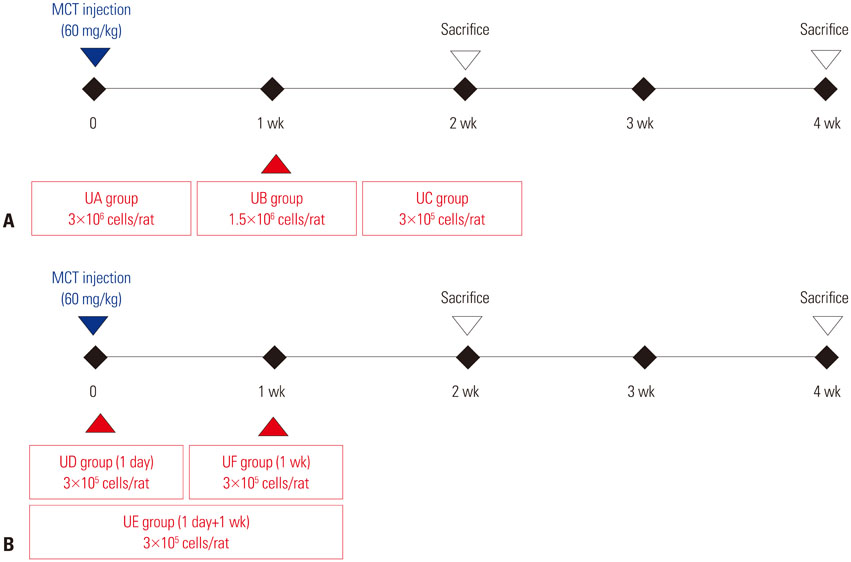
This study was divided into two parts. The first part is an investigation of dose-dependent effect. hUCB-MSCs were administered into 3 groups of rats (UA: 3×10ⶠcells, UB: 1.5×10ⶠcells, UC: 3×10ⵠcells) via the external jugular vein at week 1 after monocrotaline (MCT) injection. The second part is a search for optimal treatment timing in 3×10ⵠcells dose of hUCB-MSCs administered at day 1 for UD group (low dose of hUCB-MSCs at day 1), at day 1 and week 1 for the UE group (dual transfusion of low dose of hUCB-MSCs at day 1 and week 1) and at 1 week for the UF group (reversal treatment of low dose hUCB-MSC at week 1) after MCT injection.
RESULTS
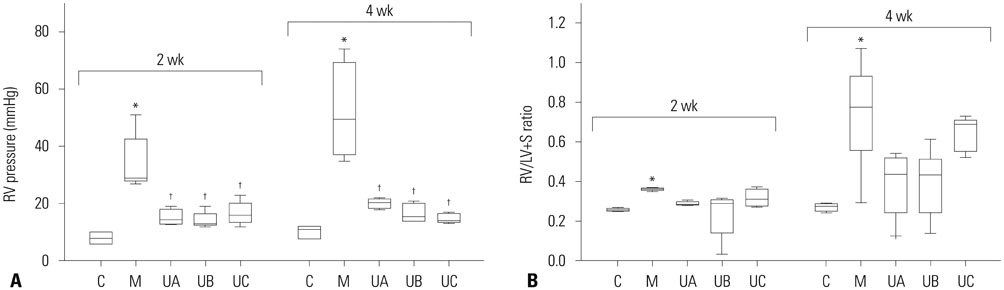
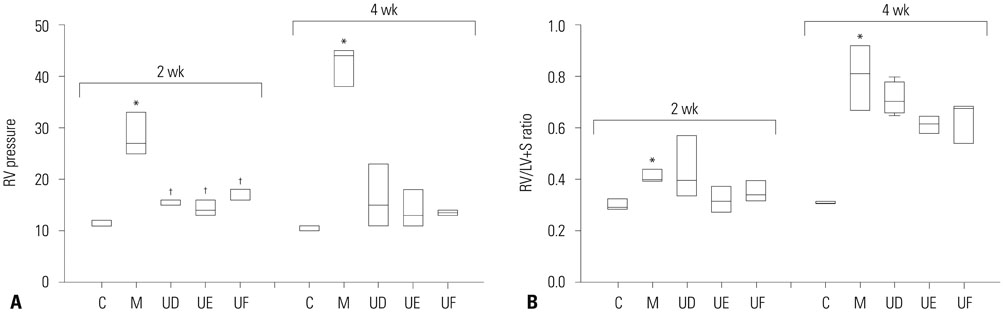
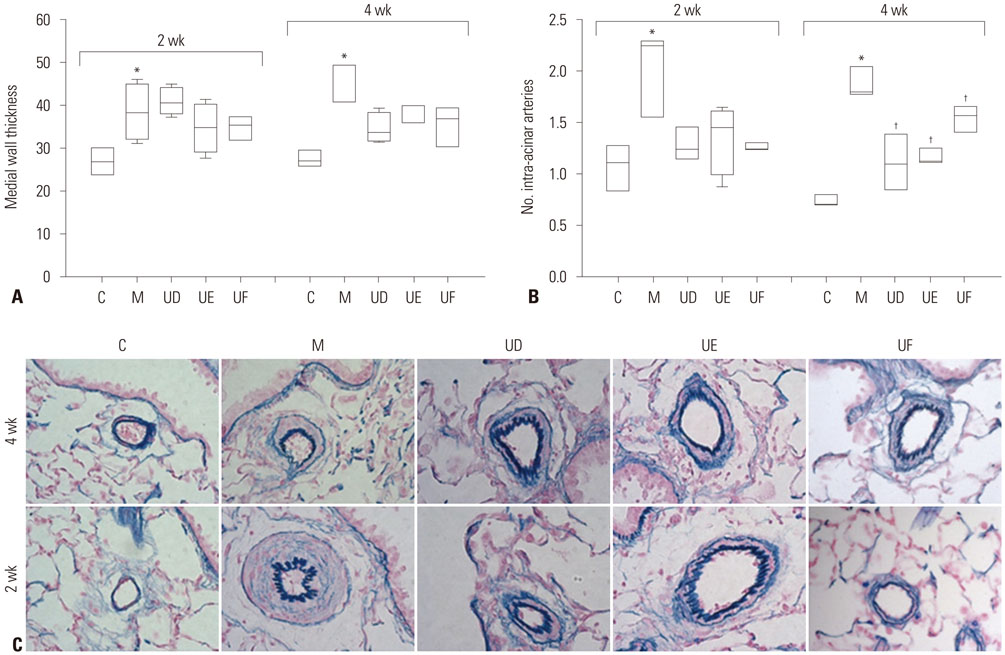
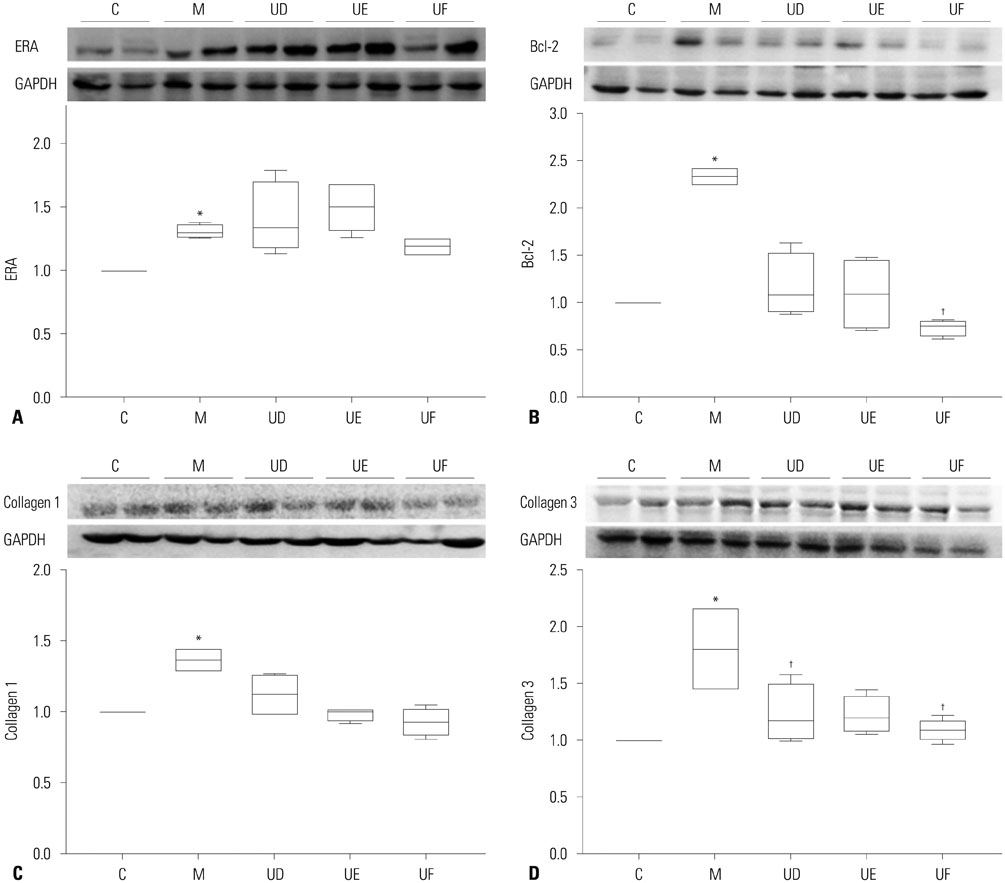
The administration of 3×10ⵠhUCB-MSCs was as effective as the 3×10ⶠdose in decreasing mean right ventricle (RV) pressure and pulmonary pathological changes. Early treatment with hUCB-MSCs improved mean RV pressure, pulmonary pathological changes and heart collagen 3 protein expression levels in PAH.
CONCLUSION
Low-dose early treatment of hUCB-MSCs is as effective as a high dose treatment of hUCB-MSCs in improving PAH although dual or reversal treatment is still more effective.
MeSH Terms
-
Animals
Disease Models, Animal
Familial Primary Pulmonary Hypertension
Humans
Hypertension, Pulmonary/chemically induced/*therapy
Hypertrophy, Right Ventricular/physiopathology
Male
*Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/*cytology/metabolism
Monocrotaline/toxicity
Pulmonary Artery/pathology
Rats
Rats, Sprague-Dawley
Time Factors
Monocrotaline
Figure
Cited by 1 articles
-
Stem Cell and Exosome Therapy in Pulmonary Hypertension
Seyeon Oh, Ji-Hye Jung, Kyung-Jin Ahn, Albert Youngwoo Jang, Kyunghee Byun, Phillip C. Yang, Wook-Jin Chung
Korean Circ J. 2022;52(2):110-122. doi: 10.4070/kcj.2021.0191.
Reference
-
1. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013; 62:25 Suppl. D34–D41.
Article2. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012; 122:4306–4313.
Article3. Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmüller P, et al. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation. 2014; 129:1332–1340.
Article4. Price LC, Wort SJ, Perros F, Dorfmüller P, Huertas A, Montani D, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012; 141:210–221.
Article5. Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004; 351:1425–1436.
Article6. Lee H, Lee JC, Kwon JH, Kim KC, Cho MS, Yang YS, et al. The effect of umbilical cord blood derived mesenchymal stem cells in monocrotaline-induced pulmonary artery hypertension rats. J Korean Med Sci. 2015; 30:576–585.
Article7. Kim KC, Lee JC, Lee H, Cho MS, Choi SJ, Hong YM. Changes in caspase-3, B cell leukemia/lymphoma-2, interleukin-6, tumor necrosis factor-α and vascular endothelial growth factor gene expression after human umbilical cord blood derived mesenchymal stem cells transfusion in pulmonary hypertension rat models. Korean Circ J. 2016; 46:79–92.
Article8. Li J, Li D, Liu X, Tang S, Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPSinduced acute lung injury in rats. J Inflamm (Lond). 2012; 9:33.
Article9. Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012; 2:170–181.
Article10. Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh W, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014; 164:966–972.
Article11. Zhang WG, He L, Shi XM, Wu SS, Zhang B, Mei L, et al. Regulation of transplanted mesenchymal stem cells by the lung progenitor niche in rats with chronic obstructive pulmonary disease. Respir Res. 2014; 15:33.
Article12. Jin Z, Pan X, Zhou K, Bi H, Wang L, Yu L, et al. Biological effects and mechanisms of action of mesenchymal stem cell therapy in chronic obstructive pulmonary disease. J Int Med Res. 2015; 43:303–310.
Article13. Barczyk M, Schmidt M, Mattoli S. Stem cell-based therapy in idiopathic pulmonary fibrosis. Stem Cell Rev. 2015; 11:598–620.
Article14. Weiss DJ. Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2014; 32:16–25.
Article15. Souidi N, Stolk M, Seifert M. Ischemia-reperfusion injury: beneficial effects of mesenchymal stromal cells. Curr Opin Organ Transplant. 2013; 18:34–43.16. Xue J, Li X, Lu Y, Gan L, Zhou L, Wang Y, et al. Gene-modified mesenchymal stem cells protect against radiation-induced lung injury. Mol Ther. 2013; 21:456–465.
Article17. Wannemuehler TJ, Manukyan MC, Brewster BD, Rouch J, Poynter JA, Wang Y, et al. Advances in mesenchymal stem cell research in sepsis. J Surg Res. 2012; 173:113–126.
Article18. Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007; 292:H1120–H1128.
Article19. Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012; 303:L967–L977.
Article20. Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011; 29:913–919.
Article21. Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani EH, Wagenaar GT, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009; 297:H1606–H1616.
Article22. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301.
Article23. Musina RA, Bekchanova ES, Belyavskii AV, Grinenko TS, Sukhikh GT. Umbilical cord blood mesenchymal stem cells. Bull Exp Biol Med. 2007; 143:127–131.
Article24. Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005; 32:270–279.
Article25. Lee HR, Kim TH, Choi KJ, Choi KC. Effects of octylphenol on the expression of cell cycle-related genes and the growth of mesenchymal stem cells derived from human umbilical cord blood. Int J Mol Med. 2014; 33:221–226.
Article26. Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002; 40:780–788.
Article27. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003; 100:8407–8411.
Article28. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363:1439–1441.
Article29. Gitelman SE, Haller MJ, Schatz D. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009; 302:624–625.
Article30. Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009; 301:1573–1579.
Article31. Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007; 4:e269.
Article32. Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008; 38:1745–1755.
Article33. Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005; 96:442–450.
Article34. Pierro M, Ionescu L, Montemurro T, Vadivel A, Weissmann G, Oudit G, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013; 68:475–484.
Article35. van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009; 180:1131–1142.
Article36. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Transplantation in Umbilical Cord Blood(I) Expansion Effects of Stem Cells in Umbilical Cord Blood with Various Hematopoietic Growth Factors
- Microarray analysis after umbilical cord blood derived mesenchymal stem cells injection in monocrotaline-induced pulmonary artery hypertension rats
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Stem Cell Therapy for Bronchopulmonary Dysplasia: Bench to Bedside Translation
- Establishment of High Throughput Screening System Using Human Umbilical Cord-derived Mesenchymal Stem Cells